Using dendritic cell maturation and IL-12 producing capacity as markers of function: a cautionary tale
- PMID: 18391760
- PMCID: PMC2744357
- DOI: 10.1097/CJI.0b013e318165f5d2
Using dendritic cell maturation and IL-12 producing capacity as markers of function: a cautionary tale
Abstract
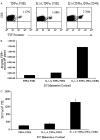
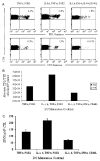
Effective dendritic cell (DC) function depends on sufficient expression of antigen and costimulatory molecules, and secretion of interleukin (IL)-12. We sought to augment DC stimulatory capacity by optimizing DC phenotype and IL-12 production. DCs, obtained by CD14-selection, were matured using 8 different cytokine cocktails, and expression of costimulatory/major histocompatibility complex molecules and IL-12 production at the end of maturation was assessed. DC stimulatory capacity was determined after pulsing with immunogenic adenoviral CD8 peptide epitopes or after transduction with an Ad5f35-null vector. Resultant T-cell cultures were analyzed using pentamer and interferon-gamma enzyme-linked immunosorbent spot assays. On the basis of DC expression of maturation markers and IL-12 production, we defined prototype "minimal" [tumor necrosis factor-alpha (TNF-alpha), prostaglandin E2], "standard" (IL-1, IL-6, TNF-alpha, prostaglandin E2), and "optimal" (IL-1, IL-6, TNF-alpha, interferon-alpha, CD40 ligand) DC cocktails. Optimal DCs were functionally superior when pulsed with CD8 peptides, but when transduced with Ad5f35, functioned poorly as antigen-presenting cells. We investigated the mechanisms underlying this discrepancy and suggest that prolonged stimulation with potent cytokines (optimal cocktail) in combination with adenoviral transduction alters the kinetics of DC maturation such that the DCs are functionally exhausted by the traditional 48-hour maturation time point. Shortening the DC maturation period posttransduction restored optimal DC stimulatory capacity. Thus, maturation stimuli and viral transduction affects DC phenotype, IL-12 producing capacity, and kinetics of maturation, and all must be considered before designing protocols to generate the optimal DC for cytotoxic T lymphocyte generation.
Figures






References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Bollard CM, Straathof KC, Huls MH, et al. The generation and characterization of LMP2-specific CTLs for use as adoptive transfer from patients with relapsed EBV-positive Hodgkin disease. J Immunother (1997) 2004;27:317–327. - PubMed
-
- Mackensen A, Meidenbauer N, Vogl S, et al. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol. 2006;24:5060–5069. - PubMed
-
- Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. - PubMed
-
- Figdor CG, de Vries IJ, Lesterhuis WJ, et al. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

