Progression of intracranial glioma disrupts thymic homeostasis and induces T-cell apoptosis in vivo
- PMID: 18392618
- PMCID: PMC11030257
- DOI: 10.1007/s00262-008-0508-3
Progression of intracranial glioma disrupts thymic homeostasis and induces T-cell apoptosis in vivo
Abstract
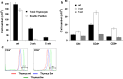
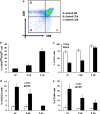
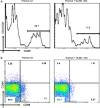
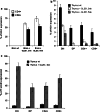
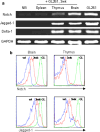
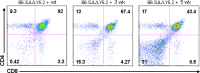
The thymus is the site where all T-cell precursors develop, mature, and subsequently leave as mature T-cells. Since the mechanisms that mediate and regulate thymic apoptosis are not fully understood, we utilized a syngenic GL261 murine glioma model to further elucidate the fate of T-cells in tumor bearing C57BL/6 mice. First, we found a dramatic reduction in the size of the thymus accompanied by a decrease in thymic cellularity in response to glioma growth in the brains of affected mice. There was a marked reduction of double positive subset and an increase in the frequency of CD4(+) and CD8(+) single positive T-cell subsets. Analysis of double negative thymocytes showed an increase in the accumulation of CD44(+) cells. In contrast, there was a marked loss of CD44 and CD122 expression in CD4(+) and CD8(+) subsets. The growth of intracranial tumors was also associated with decreased levels of HO-1, a mediator of anti-apoptotic function, and increased levels of Notch-1 and its ligand, Jagged-1. To determine whether thymic atrophy could be due to the effect of Notch and its ligand expression by glioma in vivo, we performed a bone marrow transplant experiment. Our results suggest that Notch-1 and its ligand Jagged-1 can induce apoptosis of thymocytes, thereby influencing thymic development, immune system homeostasis, and function of the immune cells in a model of experimental glioma.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

