Vascular biology and pathobiology of the liver: Report of a single-topic symposium
- PMID: 18393322
- PMCID: PMC2724750
- DOI: 10.1002/hep.22203
Vascular biology and pathobiology of the liver: Report of a single-topic symposium
Abstract
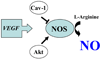
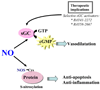
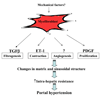
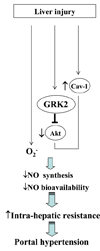
Portal hypertension and its complications account for the majority of morbidity and mortality that occurs in patients with cirrhosis. In addition to portal hypertension, a number of other vascular syndromes are also of great importance, especially the ischemia-reperfusion (IR) injury. With the identification of major vascular defects that could account for many of the clinical sequelae of these syndromes, the liver vasculature field has now integrated very closely with the broader vascular biology discipline. In that spirit, the Henry and Lillian Stratton Basic Research Single Topic Conference was held on the topic of Vascular Biology and Pathobiology of the Liver. The course took place approximately 10 years after the first American Association for the Study of Liver Disease (AASLD)-sponsored conference on this topic that occurred in Reston, Virginia. The conference initiated with an introduction to basic vascular cell signaling and then explored vascular biology specifically as it relates to liver cells. Subsequently, specific disease syndromes were discussed in more detail including portal hypertension and IR injury. Finally, clinical and translational sessions focused on emerging therapies and technologies to treat vascular diseases of the liver.
Figures

Similar articles
-
The paradox of nitric oxide in cirrhosis and portal hypertension: too much, not enough.Hepatology. 2002 Feb;35(2):478-91. doi: 10.1053/jhep.2002.31432. Hepatology. 2002. PMID: 11826425 Review. No abstract available.
-
Vascular pathobiology in chronic liver disease and cirrhosis - current status and future directions.J Hepatol. 2014 Oct;61(4):912-24. doi: 10.1016/j.jhep.2014.05.047. Epub 2014 Jun 6. J Hepatol. 2014. PMID: 24911462 Free PMC article. Review.
-
Nitric oxide and portal hypertension: its role in the regulation of intrahepatic and splanchnic vascular resistance.Semin Liver Dis. 1999;19(4):411-26. doi: 10.1055/s-2007-1007129. Semin Liver Dis. 1999. PMID: 10643626 Review.
-
[Hyperdynamic circulation in patients with liver cirrhosis and portal hypertension].Korean J Gastroenterol. 2009 Sep;54(3):143-8. doi: 10.4166/kjg.2009.54.3.143. Korean J Gastroenterol. 2009. PMID: 19844149 Review. Korean.
-
Graft injury in relation to graft size in right lobe live donor liver transplantation: a study of hepatic sinusoidal injury in correlation with portal hemodynamics and intragraft gene expression.Ann Surg. 2003 Feb;237(2):256-64. doi: 10.1097/01.SLA.0000048976.11824.67. Ann Surg. 2003. PMID: 12560784 Free PMC article.
Cited by
-
Liver fibrosis: Pathophysiology and clinical implications.WIREs Mech Dis. 2021 Jan;13(1):e1499. doi: 10.1002/wsbm.1499. Epub 2020 Jul 26. WIREs Mech Dis. 2021. PMID: 32713091 Free PMC article. Review.
-
Hepatic dimethylarginine-dimethylaminohydrolase1 is reduced in cirrhosis and is a target for therapy in portal hypertension.J Hepatol. 2015 Feb;62(2):325-31. doi: 10.1016/j.jhep.2014.08.024. Epub 2014 Aug 23. J Hepatol. 2015. PMID: 25152204 Free PMC article.
-
Cyclic GMP in Liver Cirrhosis-Role in Pathophysiology of Portal Hypertension and Therapeutic Implications.Int J Mol Sci. 2021 Sep 26;22(19):10372. doi: 10.3390/ijms221910372. Int J Mol Sci. 2021. PMID: 34638713 Free PMC article. Review.
-
Pathophysiology of portal hypertension.Clin Liver Dis. 2014 May;18(2):281-91. doi: 10.1016/j.cld.2013.12.001. Epub 2014 Feb 25. Clin Liver Dis. 2014. PMID: 24679494 Free PMC article. Review.
-
Hepatic sinusoids in liver injury, inflammation, and fibrosis: new pathophysiological insights.J Gastroenterol. 2016 Jun;51(6):511-9. doi: 10.1007/s00535-016-1190-4. Epub 2016 Mar 3. J Gastroenterol. 2016. PMID: 26939970 Review.
References
-
- Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
