Beta-catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse development
- PMID: 18393386
- PMCID: PMC4449338
- DOI: 10.1002/hep.22225
Beta-catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse development
Abstract
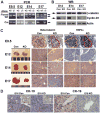
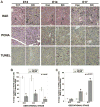
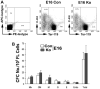
Beta-catenin, the central component of the canonical Wnt pathway, plays important roles in the processes of liver regeneration, growth, and cancer. Previously, we identified temporal expression of beta-catenin during liver development. Here, we characterize the hepatic phenotype, resulting from the successful deletion of beta-catenin in the developing hepatoblasts utilizing Foxa3-cyclization recombination and floxed-beta-catenin (exons 2 through 6) transgenic mice. Beta-catenin loss in developing livers resulted in significantly underdeveloped livers after embryonic day 12 (E12) with lethality occurring at around E17 stages. Histology revealed an overall deficient hepatocyte compartment due to (1) increased cell death due to oxidative stress and apoptosis, and (2) diminished expansion secondary to decreased cyclin-D1 and impaired proliferation. Also, the remnant hepatocytes demonstrated an immature phenotype as indicated by high nuclear to cytoplasmic ratio, poor cell polarity, absent glycogen, and decreased expression of key liver-enriched transcription factors: CCAAT-enhancer binding protein-alpha and hepatocyte nuclear factor-4alpha. A paucity of primitive bile ducts was also observed. While the stem cell assays demonstrated no intrinsic defect in hematopoiesis, distorted hepatic architecture and deficient hepatocyte compartments resulted in defective endothelial cell organization leading to overall fetal pallor.
Conclusion: Beta-catenin regulates multiple, critical events during the process of hepatic morphogenesis, including hepatoblast maturation, expansion, and survival, making it indispensable to survival.
Conflict of interest statement
Potential conflict of interest: Nothing to report.
Figures




References
-
- Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–3537. - PubMed
-
- Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science. 2000;287:1606–1609. - PubMed
-
- Morin PJ. beta-Catenin signaling and cancer. Bioessays. 1999;21:1021–1030. - PubMed
-
- Pennisi E. How a growth control path takes a wrong turn to cancer. Science. 1998;281:1438–1439. News; Comment. erratum appears in Science 1998;281:1809. - PubMed
-
- Barker N, Morin PJ, Clevers H. The Yin-Yang of TCF/beta-catenin signaling. Adv Cancer Res. 2000;77:1–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
