Vascularization of engineered tissues: approaches to promote angio-genesis in biomaterials
- PMID: 18393893
- PMCID: PMC3929210
- DOI: 10.2174/156802608783790983
Vascularization of engineered tissues: approaches to promote angio-genesis in biomaterials
Abstract
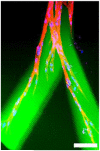
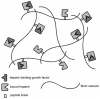
Although there have been extensive research efforts to create functional tissues and organs, most successes in tissue engineering have been limited to avascular or thin tissues. The major hurdle in development of more complex tissues lies in the formation of vascular networks capable of delivering oxygen and nutrients throughout the engineered constructs. Sufficient neovascularization in scaffold materials can be achieved through coordinated application of angiogenic factors with proper cell types in biomaterials. This review present the current research developments in the design of biomaterials and their biochemical and biochemical modifications to produce vascularized tissue constructs.
Figures





References
-
- Jain RK, Au P, Tam J, Duda DG, Fukumura D. Engineering vascularized tissue. Nat. Biotechnol. 2005;23:821–823. - PubMed
-
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. - PubMed
-
- Naughton GK. From lab bench to market: critical issues in tissue engineering. Ann. N. Y. Acad. Sci. 2002;961:372–385. - PubMed
-
- Oberpenning F, Meng J, Yoo JJ, Atala A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat. Biotechnol. 1999;17:149–155. - PubMed
-
- Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

