Expression of integrin alphavbeta3 in gliomas correlates with tumor grade and is not restricted to tumor vasculature
- PMID: 18394009
- PMCID: PMC2607528
- DOI: 10.1111/j.1750-3639.2008.00137.x
Expression of integrin alphavbeta3 in gliomas correlates with tumor grade and is not restricted to tumor vasculature
Abstract
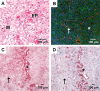
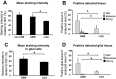
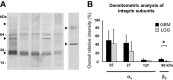
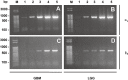
In malignant gliomas, the integrin adhesion receptors seem to play a key role for invasive growth and angiogenesis. However, there is still a controversy about the expression and the distribution of alpha(v)beta(3) integrin caused by malignancy. The aim of our study was to assess the extent and pattern of alpha(v)beta(3) integrin expression within primary glioblastomas (GBMs) compared with low-grade gliomas (LGGs). Tumor samples were immunostained for the detection of alpha(v)beta(3) integrin and quantified by an imaging software. The expression of alpha(v)beta(3) was found to be significantly higher in GBMs than in LGGs, whereby focal strong reactivity was restricted to GBMs only. Subsequent analysis revealed that not only endothelial cells but also, to a large extent, glial tumor cells contribute to the overall amount of alpha(v)beta(3) integrin in the tumors. To further analyze the integrin subunits, Western blots from histologic sections were performed, which demonstrated a significant difference in the expression of the beta(3) integrin subunit between GBMs and LGGs. The presented data lead to new insights in the pattern of alpha(v)beta(3) integrin in gliomas and are of relevance for the inhibition of alpha(v)beta(3) integrin with specific RGD peptides and interfering drugs to reduce angiogenesis and tumor growth.
Figures

References
-
- Bader BL, Rayburn H, Crowley D, Hynes RO (1998) Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell 95:507–519. - PubMed
-
- Beer AJ, Schwaiger M (2007) Molecular imaging with new PET tracers. Radiologe 47:8–17. - PubMed
-
- Beer AJ, Haubner R, Goebel M, Luderschmidt S, Spilker ME, Wester HJ et al (2005) Biodistribution and pharmacokinetics of the alphavbeta3‐selective tracer 18F‐galacto‐RGD in cancer patients. J Nucl Med 46:1333–1341. - PubMed
-
- Beer AJ, Haubner R, Sarbia M, Goebel M, Luderschmidt S, Grosu AL et al (2006) Positron emission tomography using [18F] Galacto‐RGD identifies the level of integrin alpha(v)beta3 expression in man. Clin Cancer Res 12:3942–3949. - PubMed
-
- Bello L, Francolini M, Marthyn P, Zhang J, Carroll RS, Nikas DC et al (2001) Alpha(v)beta3 and alpha(v)beta5 integrin expression in glioma periphery. Neurosurgery 49:380–389. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

