Retrospective analysis of monkeypox infection
- PMID: 18394277
- PMCID: PMC2570942
- DOI: 10.3201/eid1404.071044
Retrospective analysis of monkeypox infection
Abstract
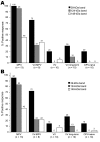
Serologic cross-reactivity between orthopoxviruses is a substantial barrier to laboratory diagnosis of specific orthopoxvirus infections and epidemiologic characterization of disease outbreaks. Historically, time-consuming and labor-intensive strategies such as cross-adsorbed neutralization assays, immunofluorescence assays, and hemagglutination-inhibition assays have been used to identify orthopoxvirus infections. We used cross-adsorption to develop a simple and quantitative postadsorption ELISA for distinguishing between monkeypox and vaccinia infections. Despite the difficulty of diagnosing clinically inapparent monkeypox in previously vaccinated persons, this technique exhibited 100% sensitivity and 100% specificity for identifying clinically overt monkeypox infection irrespective of vaccination history. We also describe a Western blot technique in which up to 3 diagnostic bands may be used to distinguish between vaccinia and monkeypox infection. The techniques described provide independent diagnostic tests suitable for retrospective analysis of monkeypox outbreaks.
Figures


References
-
- Jezek Z, Fenner F, eds. Human monkeypox. Basel: Karger; 1988.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
