Wild ducks as long-distance vectors of highly pathogenic avian influenza virus (H5N1)
- PMID: 18394278
- PMCID: PMC2570914
- DOI: 10.3201/eid1404.071016
Wild ducks as long-distance vectors of highly pathogenic avian influenza virus (H5N1)
Abstract
Wild birds have been implicated in the expansion of highly pathogenic avian influenza virus (H5N1) outbreaks across Asia, the Middle East, Europe, and Africa (in addition to traditional transmission by infected poultry, contaminated equipment, and people). Such a role would require wild birds to excrete virus in the absence of debilitating disease. By experimentally infecting wild ducks, we found that tufted ducks, Eurasian pochards, and mallards excreted significantly more virus than common teals, Eurasian wigeons, and gadwalls; yet only tufted ducks and, to a lesser degree, pochards became ill or died. These findings suggest that some wild duck species, particularly mallards, can potentially be long-distance vectors of highly pathogenic avian influenza virus (H5N1) and that others, particularly tufted ducks, are more likely to act as sentinels.
Figures
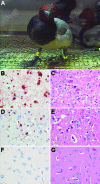

References
-
- World Health Organization. Epidemic and pandemic alert and response. Avian influenza [cited 2008 22 Jan]. Available from http://www.who.int/csr/disease/avian_influenza/en/index.html
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
