Olfactory memory traces in Drosophila
- PMID: 18394482
- PMCID: PMC2692900
- DOI: 10.1016/S0079-6123(07)00018-0
Olfactory memory traces in Drosophila
Abstract
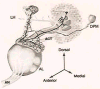
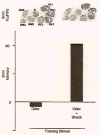
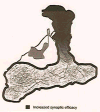
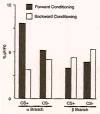
In Drosophila, the fruit fly, coincident exposure to an odor and an aversive electric shock can produce robust behavioral memory. This behavioral memory is thought to be regulated by cellular memory traces within the central nervous system of the fly. These molecular, physiological, or structural changes in neurons, induced by pairing odor and shock, regulate behavior by altering the neurons' response to the learned environment. Recently, novel in vivo functional imaging techniques have allowed researchers to observe cellular memory traces in intact animals. These investigations have revealed interesting temporal and spatial dynamics of cellular memory traces. First, a short-term cellular memory trace was discovered that exists in the antennal lobe, an early site of olfactory processing. This trace represents the recruitment of new synaptic activity into the odor representation and forms for only a short period of time just after training. Second, an intermediate-term cellular memory trace was found in the dorsal paired medial neuron, a neuron thought to play a role in stabilizing olfactory memories. Finally, a long-term protein synthesis-dependent cellular memory trace was discovered in the mushroom bodies, a structure long implicated in olfactory learning and memory. Therefore, it appears that aversive olfactory associations are encoded by multiple cellular memory traces that occur in different regions of the brain with different temporal domains.
Figures
References
-
- Cheng Y, Endo K, Wu K, Rodan AR, Heberlein U, Davis RL. Drosophila fasciclin II is required for the formation of odor memories and for normal sensitivity to alcohol. Cell. 2001;105:757–768. - PubMed
-
- Davis RL. Olfactory learning. Neuron. 2004;44:31–48. - PubMed
-
- Davis RL. Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu Rev Neurosci. 2005;28:275–302. - PubMed
-
- de Belle JS, Heisenberg M. Associate odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. - PubMed
-
- Dubnau J, Grady L, Kitamoto T, Tully T. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature. 2001;411:476–480. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

