Multiple roles for Med12 in vertebrate endoderm development
- PMID: 18394596
- PMCID: PMC2435012
- DOI: 10.1016/j.ydbio.2008.02.031
Multiple roles for Med12 in vertebrate endoderm development
Abstract
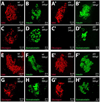
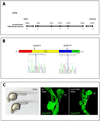
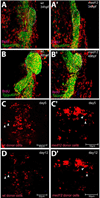
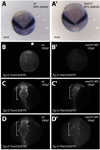
In zebrafish, the endoderm originates at the blastula stage from the most marginal blastomeres. Through a series of complex morphogenetic movements and differentiation events, the endodermal germ layer gives rise to the epithelial lining of the digestive tract as well as its associated organs such as the liver, pancreas, and swim bladder. How endodermal cells differentiate into distinct cell types such as hepatocytes or endocrine and exocrine pancreatic cells remains a major question. In a forward genetic screen for genes regulating endodermal organ development, we identified mutations at the shiri locus that cause defects in the development of a number of endodermal organs including the liver and pancreas. Detailed phenotypic analyses indicate that these defects are partially due to a reduction in endodermal expression of the hairy/enhancer of split-related gene, her5, at mid to late gastrulation stages. Using the Tg(0.7her5:EGFP)(ne2067) line, we show that her5 is expressed in the endodermal precursors that populate the pharyngeal region as well as the organ-forming region. We also find that knocking down her5 recapitulates some of the endodermal phenotypes of shiri mutants, further revealing the role of her5 in endoderm development. Positional cloning reveals that shiri encodes Med12, a regulatory subunit of the transcriptional Mediator complex recently associated with two human syndromes. Additional studies indicate that Med12 modulates the ability of Casanova/Sox32 to induce sox17 expression. Thus, detailed phenotypic analyses of embryos defective in a component of the Mediator complex have revealed new insights into discrete aspects of vertebrate endoderm development, and provide possible explanations for the craniofacial and digestive system defects observed in humans with mutations in MED12.
Figures


References
-
- Alexander J, Stainier DY, Yelon D. Screening mosaic F1 females for mutations affecting zebrafish heart induction and patterning. Dev Genet. 1998;22:288–299. - PubMed
-
- Alexander J, Rothenberg M, Henry GL, Stainier DY. Casanova plays an early and essential role in endoderm formation in zebrafish. Dev Biol. 1999;215:343–357. - PubMed
-
- Aoki TO, Mathieu J, Saint-Etienne L, Rebagliati MR, Peyrieras N, Rosa FM. Regulation of nodal signaling and mesendoderm formation by TARAM-A, a TGFbeta-related type I receptor. Dev Biol. 2002;241:273–288. - PubMed
-
- Biemar F, Argenton F, Schmidtke R, Epperlein S, Peers B, Driever W. Pancreas development in zebrafish: early dispersed appearance of endocrine hormone expressing cells and their convergence to form the definitive islet. Dev Biol. 2001;230:189–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

