Human neural stem cells migrate along the nigrostriatal pathway in a primate model of Parkinson's disease
- PMID: 18394605
- PMCID: PMC2483423
- DOI: 10.1016/j.expneurol.2008.01.025
Human neural stem cells migrate along the nigrostriatal pathway in a primate model of Parkinson's disease
Abstract
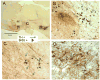
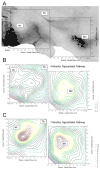
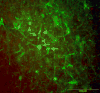
Although evidence of damage-directed neural stem cell (NSC) migration has been well-documented in the rodent, to our knowledge it has never been confirmed or quantified using human NSC (hNSC) in an adult non-human primate modeling a human neurodegenerative disease state. In this report, we attempt to provide that confirmation, potentially advancing basic stem cell concepts toward clinical relevance. hNSCs were implanted into the caudate nucleus (bilaterally) and substantia nigra (unilaterally) of 7, adult St. Kitts African green monkeys (Chlorocebus sabaeus) with previous exposure to systemic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a neurotoxin that disrupts the dopaminergic nigrostriatal pathway. A detailed quantitative analysis of hNSC migration patterns at two time points (4 and 7 months) following transplantation was performed. Density contour mapping of hNSCs along the dorsal-ventral and medial-lateral axes of the brain suggested that >80% of hNSCs migrated from the point of implantation to and along the impaired nigrostriatal pathway. Although 2/3 of hNSCs were transplanted within the caudate, <1% of 3x10(6) total injected donor cells were identified at this site. The migrating hNSC did not appear to be pursuing a neuronal lineage. In the striatum and nigrostriatal pathway, but not in the substantia nigra, some hNSCs were found to have taken a glial lineage. The property of neural stem cells to align themselves along a neural pathway rendered dysfunctional by a given disease is potentially a valuable clinical tool.
Figures



References
-
- An YH, Wang HY, Gao ZX, Wang ZC. Differentiation of rat neural stem cells and its relationship with environment. Biomed Environ Sci. 2004;17:1–7. - PubMed
-
- Behrstock S, Ebert A, McHugh J, Vosberg S, Moore J, Schneider B, Capowski E, Hei D, Kordower J, Aebischer P, Svendsen CN. Human neural progenitors deliver glial cell line-derived neurotrophic factor to parkinsonian rodents and aged primates. Gene Ther. 2006;13:379–88. - PubMed
-
- Bezard E, Gross CE. Compensatory mechanisms in experimental and human parkinsonism: Towards a dynamic approach. Prog Neurobiol. 1998;55:93–116. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

