Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II study
- PMID: 18394689
- PMCID: PMC2692926
- DOI: 10.1016/j.ygyno.2008.02.024
Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II study
Abstract
Objective: Doxorubicin-based treatment is standard therapy for metastatic uterine leiomyosarcoma. There is no standard second-line therapy. We determined activity of fixed-dose rate gemcitabine plus docetaxel as second-line treatment for metastatic uterine leiomyosarcoma.
Methods: Eligible women with unresectable uterine leiomyosarcoma progressing after prior cytotoxic therapy were treated with gemcitabine 900 mg/m(2) days one and eight over 90 min, plus docetaxel 100 mg/m(2) on day 8 of a 21-day cycle with granulocyte growth factor. Patients with prior pelvic radiation received lower doses. Response Evaluation Criteria in Solid Tumors (RECIST) response was assessed by computed tomography (CT).
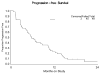
Results: Forty-eight of 51 women were evaluable for response (one wrong histology, two never treated). Prior therapy was doxorubicin-based in 90%, and ifosfamide-based in 6%. The overall objective response rate is 27%, with complete response in 6.3% (3/48), and partial response in 20.8% (10/48). An additional 50% (24/48) had stable disease (median duration 5.4 months). The median number of cycles per patient was 5.5 (range 1-22); 73% of patients remained progression-free at 12 weeks and 52% at 24 weeks. The predominant toxicity was uncomplicated myelosuppression: thrombocytopenia grade 3 (29%), grade 4 (10.4%); neutropenia grade 3 (12.5%), grade 4 (8.3%) anemia grade 3 (20.8%), grade 4 (4.2%). While pulmonary toxicity was reported, no patient had drug-related pneumonitis/hypoxia-type toxicity. Median progression-free survival (PFS) was 5.6+ months (range 0.7-27+ months). The median duration of objective response was 9+ months (range 3.9-24.5+ months).
Conclusion: Fixed-dose rate gemcitabine plus docetaxel is active second-line therapy for uterine leiomyosarcoma.
Conflict of interest statement
Figures
References
-
- Major FJ, Blessing JA, Silverberg SG, Morrow CP, Creasman WT, Currie JL, et al. Prognostic factors in early-stage uterine sarcoma. Cancer. 1993;71:1702–9. - PubMed
-
- Levenback C, Rubin SC, McCormack PM, Hoskins WJ, Atkinson EN, Lewis JL., Jr Resection of pulmonary metastases from uterine sarcomas. Gynecol Oncol. 1992;45:202–5. - PubMed
-
- Thigpen JT, Blessing JA, Beecham J, Homesley H, Yordan E. A Phase II trial of cisplatin as first-line chemotherapy in patients with advanced or recurrent uterine sarcomas: a Gynecologic Oncology Group study. J Clin Oncol. 1991;9:1962–6. - PubMed
-
- Thigpen JT, Blessing JA, Wilbanks GD. Cisplatin as second-line chemotherapy in the treatment of advanced or recurrent leiomyosarcoma of the uterus. A phase II trial of the Gynecologic Oncology Group. Am J Clin Oncol. 1986;9:18–20. - PubMed
-
- Muss HB, Bundy BN, Adcock L, Beecham J. Mitoxantrone in the treatment of advanced uterine sarcoma: A phase II trial of the Gynecologic Oncology Group. Am J Clin Oncol. 1990;13:32–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical