Use of 18O labels to monitor deamidation during protein and peptide sample processing
- PMID: 18394920
- PMCID: PMC3105245
- DOI: 10.1016/j.jasms.2008.02.011
Use of 18O labels to monitor deamidation during protein and peptide sample processing
Abstract
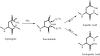
Nonenzymatic deamidation of asparagine residues in proteins generates aspartyl (Asp) and isoaspartyl (isoAsp) residues via a succinimide intermediate in a neutral or basic environment. Electron capture dissociation (ECD) can differentiate and quantify the relative abundance of these isomeric products in the deamidated proteins. This method requires the proteins to be digested, usually by trypsin, into peptides that are amenable to ECD. ECD of these peptides can produce diagnostic ions for each isomer; the c. + 58 and z - 57 fragment ions for the isoAsp residue and the fragment ion ((M + nH)((n-1)+.) - 60) corresponding to the side-chain loss from the Asp residue. However, deamidation can also occur as an artifact during sample preparation, particularly when using typical tryptic digestion protocols. With 18O labeling, it is possible to differentiate deamidation occurring during trypsin digestion which causes a +3 Da (18O1 + 1D) mass shift from the pre-existing deamidation, which leads to a +1-Da mass shift. This paper demonstrates the use of (18)O labeling to monitor three rapidly deamidating peptides released from proteins (calmodulin, ribonuclease A, and lysozyme) during the time course of trypsin digestion processes, and shows that the fast (approximately 4 h) trypsin digestion process generates no additional detectable peptide deamidations.
Figures





References
-
- Robinson NE, Robinson AB. Molecular clocks: Deamidation of asparaginyl and glutaminyl residues in peptides and proteins. Althouse Press; Cave Junction, OR: 2004.
-
- Lindner H, Sarg B, Hoertnagl B, Helliger W. The microheterogeneity of the mammalian H1(0) histone - Evidence for an age-dependent deamidation. J. Biol. Chem. 1998;273:13324–13330. - PubMed
-
- Bischoff R, Kolbe HV. Deamidation of asparagine and glutamine residues in proteins and peptides - structural determinants and analytical methodology. J. Chromatogr. B Biomed. Appl. 1994;662:261–278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

