NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy
- PMID: 18394936
- PMCID: PMC3528789
- DOI: 10.1016/j.immuni.2008.02.016
NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy
Erratum in
- Immunity. 2008 May;28(5):723. Greenberg, Norman R [corrected to Greenberg, Norman M]
Abstract
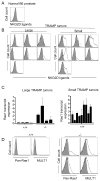
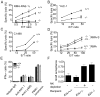
Ligands for the NKG2D stimulatory receptor are frequently upregulated on tumor lines, rendering them sensitive to natural killer (NK) cells, but the role of NKG2D in tumor surveillance has not been addressed in spontaneous cancer models. Here, we provided the first characterization of NKG2D-deficient mice, including evidence that NKG2D was not necessary for NK cell development but was critical for immunosurveillance of epithelial and lymphoid malignancies in two transgenic models of de novo tumorigenesis. In both models, we detected NKG2D ligands on the tumor cell surface ex vivo, providing needed evidence for ligand expression by primary tumors. In a prostate cancer model, aggressive tumors arising in NKG2D-deficient mice expressed higher amounts of NKG2D ligands than did similar tumors in wild-type mice, suggesting an NKG2D-dependent immunoediting of tumors in this model. These findings provide important genetic evidence for surveillance of primary tumors by an NK receptor.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures



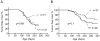
Comment in
-
Cancer immunosurveillance: NKG2D breaks cover.Immunity. 2008 Apr;28(4):492-4. doi: 10.1016/j.immuni.2008.03.007. Immunity. 2008. PMID: 18400193 Review.
References
-
- Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD, Brinster RL. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. - PubMed
-
- Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. - PubMed
-
- Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. - PubMed
-
- Bix M, Liao NS, Zijlstra M, Loring J, Jaenisch R, Raulet D. Rejection of class I MHC-deficient hemopoietic cells by irradiated MHC-matched mice. Nature. 1991;349:329–331. - PubMed
-
- Cerwenka A, Bakker ABH, McClanahan T, Wagner J, Wu J, Phillips JH, Lanier LL. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–727. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

