Opioidergic and dopaminergic modulation of respiration
- PMID: 18394974
- PMCID: PMC2642894
- DOI: 10.1016/j.resp.2008.02.004
Opioidergic and dopaminergic modulation of respiration
Abstract
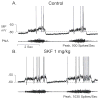
Opioids, dopamine and their receptors are present in many regions of the bulbar respiratory network. The physiological importance of endogenous opioids to respiratory control has not been explicitly demonstrated. Nonetheless, studies of opioidergic respiratory mechanisms are important because synthetic opiate drugs have respiratory side effects that in some situations pose health risks and limit their therapeutic usefulness. They can depress breathing depth and rate, blunt respiratory responsiveness to CO2 and hypoxia, increase upper airway resistance and reduce pulmonary compliance. The opiate respiratory disturbances are mainly due to agonist activation of mu- and delta-subtypes of receptor and involve specific types of respiratory-related neurons in the ventrolateral medulla and the dorsolateral pons. Endogenous dopaminergic modulation in the CNS and carotid bodies enhances CO2-dependent respiratory drive and depresses hypoxic drive. In the CNS, synthetic agonists with selectivity for D1-and D4-types of receptor slow respiratory rhythm, whereas D2-selective agonists modulate acute and chronic responses to hypoxia. D1-receptor agonists also act centrally to increase respiratory responsiveness to CO2, and counteract opiate blunting of CO2-dependent respiratory drive and depression of breathing. Cellular targets and intracellular mechanisms responsible for opioidergic and dopaminergic respiratory effects for the most part remain to be determined.
Figures


Similar articles
-
Mu Receptors.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31855381 Free Books & Documents.
-
D1-dopamine receptor agonists prevent and reverse opiate depression of breathing but not antinociception in the cat.Am J Physiol Regul Integr Comp Physiol. 2005 Jul;289(1):R45-51. doi: 10.1152/ajpregu.00868.2004. Epub 2005 Feb 10. Am J Physiol Regul Integr Comp Physiol. 2005. PMID: 15705800
-
Differential roles of opioid receptors in respiration, respiratory disease, and opiate-induced respiratory depression.Am Rev Respir Dis. 1990 Oct;142(4):895-909. doi: 10.1164/ajrccm/142.4.895. Am Rev Respir Dis. 1990. PMID: 2171388 Review.
-
D1/D2-dopamine receptor agonist dihydrexidine stimulates inspiratory motor output and depresses medullary expiratory neurons.Am J Physiol Regul Integr Comp Physiol. 2009 Jun;296(6):R1829-36. doi: 10.1152/ajpregu.00057.2009. Epub 2009 Mar 11. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19279296 Free PMC article.
-
Neurotransmitters in the CNS control of breathing.Respir Physiol. 1995 Sep;101(3):219-30. doi: 10.1016/0034-5687(95)00045-f. Respir Physiol. 1995. PMID: 8606995 Review.
Cited by
-
Objective Characterization of Opiate-Induced Chest Wall Rigidity.Cureus. 2020 Jun 5;12(6):e8459. doi: 10.7759/cureus.8459. Cureus. 2020. PMID: 32566433 Free PMC article.
-
Management of opioid analgesic overdose.N Engl J Med. 2012 Jul 12;367(2):146-55. doi: 10.1056/NEJMra1202561. N Engl J Med. 2012. PMID: 22784117 Free PMC article. Review. No abstract available.
-
Limb movement-induced hyperemia has a central hemodynamic component: evidence from a neural blockade study.Am J Physiol Heart Circ Physiol. 2010 Nov;299(5):H1693-700. doi: 10.1152/ajpheart.00482.2010. Epub 2010 Aug 27. Am J Physiol Heart Circ Physiol. 2010. PMID: 20802133 Free PMC article.
-
Excited Delirium and Sudden Death: A Syndromal Disorder at the Extreme End of the Neuropsychiatric Continuum.Front Physiol. 2016 Oct 13;7:435. doi: 10.3389/fphys.2016.00435. eCollection 2016. Front Physiol. 2016. PMID: 27790150 Free PMC article. Review.
-
Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans.J Appl Physiol (1985). 2010 Oct;109(4):966-76. doi: 10.1152/japplphysiol.00462.2010. Epub 2010 Jul 15. J Appl Physiol (1985). 2010. PMID: 20634355 Free PMC article. Clinical Trial.
References
-
- Arata A, Onimaru H, Homma I. Effects of CAMP on respiratory rhythm generation in brainstem-spinal cord preparation from newborn rat. Brain Res. 1993;606:193–199. - PubMed
-
- Bagosi Z, Jaszberenyi M, Bujdoso E, Szabo G, Telegdy G. The effects of endomorphins and diprotin A on striatal dopamine release induced by electrical stimulation-an in vitro superfusion study in rats. Neurochem Int. 2006;49:665–668. - PubMed
-
- Barnes BJ, Tuong C-M, Mellen NM. Functional imaging reveals respiratory network activity during hypoxic and opioid challenge in the neonate rat tilted saggital slab preparation. J Neurophysiol. 2007;87:2283–2302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

