RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice
- PMID: 18394989
- PMCID: PMC2366094
- DOI: 10.1016/j.cell.2008.02.042
RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice
Abstract
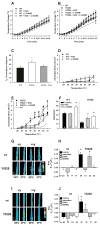
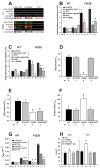
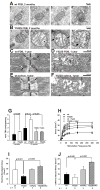
Mice with a malignant hyperthermia mutation (Y522S) in the ryanodine receptor (RyR1) display muscle contractures, rhabdomyolysis, and death in response to elevated environmental temperatures. We demonstrate that this mutation in RyR1 causes Ca(2+) leak, which drives increased generation of reactive nitrogen species (RNS). Subsequent S-nitrosylation of the mutant RyR1 increases its temperature sensitivity for activation, producing muscle contractures upon exposure to elevated temperatures. The Y522S mutation in humans is associated with central core disease. Many mitochondria in the muscle of heterozygous Y522S mice are swollen and misshapen. The mutant muscle displays decreased force production and increased mitochondrial lipid peroxidation with aging. Chronic treatment with N-acetylcysteine protects against mitochondrial oxidative damage and the decline in force generation. We propose a feed-forward cyclic mechanism that increases the temperature sensitivity of RyR1 activation and underlies heat stroke and sudden death. The cycle eventually produces a myopathy with damaged mitochondria.
Figures


Comment in
-
A SNO storm in skeletal muscle.Cell. 2008 Apr 4;133(1):33-5. doi: 10.1016/j.cell.2008.03.013. Cell. 2008. PMID: 18394987
References
-
- Aghdasi B, Reid MB, Hamilton SL. Nitric Oxide Protects the Skeletal Muscle Ca2+ Release Channel from Oxidation Induced Activation. The Journal of biological chemistry. 1997;272:25462–25467. - PubMed
-
- Aracena-Parks P, Goonasekera SA, Gilman CP, Dirksen RT, Hidalgo C, Hamilton SL. Identification of Cysteines Involved in S-Nitrosylation, S-Glutathionylation, and Oxidation to Disulfides in Ryanodine Receptor Type 1. The Journal of biological chemistry. 2006;281:40354–40368. - PubMed
-
- Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O'Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM. Nitric Oxide Regulates the Heart by Spatial Confinement of Nitric Oxide Synthase Isoforms. Nature. 2002;416:337–339. - PubMed
-
- Bellinger AM, Reiken S, Dura M, Murphy PW, Deng SX, Landry DW, Nieman D, Lehnart SE, Samaru M, Lacampagne A, Marks AR. Remodeling of Ryanodine Receptor Complex Causes “Leaky” Channels: A Molecular Mechanism for Decreased Exercise Capacity. Proceedings of the National Academy of Sciences of the United States of America.2008. - PMC - PubMed
-
- Bendahan D, Kozak-Ribbens G, Confort-Gouny S, Ghattas B, Figarella-Branger D, Aubert M, Cozzone PJ. A Noninvasive Investigation of Muscle Energetics Supports Similarities Between Exertional Heat Stroke and Malignant Hyperthermia. Anesth Analg. 2001;93:683–689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32-DE07202/DE/NIDCR NIH HHS/United States
- R01 AR041802/AR/NIAMS NIH HHS/United States
- GGP030289/TI_/Telethon/Italy
- 5P01AR052354/AR/NIAMS NIH HHS/United States
- R01 AR044657/AR/NIAMS NIH HHS/United States
- R01 AR050503/AR/NIAMS NIH HHS/United States
- R01 AR053349/AR/NIAMS NIH HHS/United States
- AR053349/AR/NIAMS NIH HHS/United States
- R29 AR044657/AR/NIAMS NIH HHS/United States
- P01 AR052354/AR/NIAMS NIH HHS/United States
- AR 050503/AR/NIAMS NIH HHS/United States
- T32 DE007202/DE/NIDCR NIH HHS/United States
- AR44657/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

