Progenitors of interstitial cells of cajal in the postnatal murine stomach
- PMID: 18395089
- PMCID: PMC2435491
- DOI: 10.1053/j.gastro.2008.01.036
Progenitors of interstitial cells of cajal in the postnatal murine stomach
Abstract
Background & aims: Maintaining the integrity of networks of interstitial cells of Cajal (ICC) is essential to preserve orderly contractile activity and neuroregulation in the gastrointestinal tract and to restore these functions after tissue damage or surgeries. Maintenance of ICC requires insulin-dependent or insulin-like growth factor I (IGF-I)-dependent production of membrane-bound stem cell factor (SCF) and may involve regeneration from local progenitors. Our goal was to identify ICC precursors in postnatal murine gastric muscles.
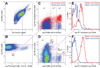
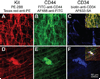
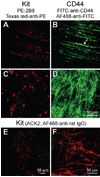
Methods: We used flow cytometry and immunohistochemistry to examine freshly dissected and cultured muscles for cells expressing CD34, an adhesion molecule expressed by stromal tumors; CD44, which occurs on mesenchymal stem cells; and receptors for SCF (Kit), insulin (Insr), and IGF-I (Igf1r). Slow waves were studied by intracellular recording.
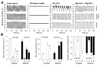
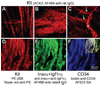
Results: In gastric muscles, we identified rare, Kit(low)CD44(+)CD34(+)Insr(+)Igf1r(+) cells resembling common embryonic precursors of ICC and smooth muscle. These putative progenitors were absent from organotypic cultures lacking mature ICC (Kit(+)CD44(+)CD34(-)Insr(-)Igf1r(-)) due to prolonged insulin/IGF-I deprivation but were rescued by IGF-I that also prevented ICC loss. Soluble SCF failed to prevent the loss of mature ICC but dramatically expanded the putative progenitors, which supported robust slow wave activity despite retaining an immature, Kit(+)CD44(+)CD34(+)Insr(+)Igf1r(+) phenotype. Differentiation of these cells into mature, network-forming ICC required IGF-I. Conversely, restoration of ICC networks by IGF-I after prolonged insulin and IGF-I deprivation required the survival of the presumed progenitors.
Conclusions: Kit(low)CD44(+)CD34(+)Insr(+)Igf1r(+) cells may be local progenitors for gastric ICC and stromal tumors. Loss of these cells may contribute to gastrointestinal dysmotilities.
Figures


Comment in
-
Progenitor cells of interstitial cells of Cajal: on the road to tissue repair.Gastroenterology. 2008 Apr;134(4):1252-4. doi: 10.1053/j.gastro.2008.02.074. Gastroenterology. 2008. PMID: 18395104 No abstract available.
References
-
- Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–343. - PubMed
-
- Huizinga JD. Gastrointestinal peristalsis: joint action of enteric nerves, smooth muscle, and interstitial cells of Cajal. Microsc Res Tech. 1999;47:239–247. - PubMed
-
- Ward SM, Sanders KM. Interstitial cells of Cajal: primary targets of enteric motor innervation. Anat Rec. 2001;262:125–135. - PubMed
-
- Fox EA, Phillips RJ, Martinson FA, et al. C-Kit mutant mice have a selective loss of vagal intramuscular mechanoreceptors in the forestomach. Anat Embryol (Berl) 2001;204:11–26. - PubMed
-
- Vanderwinden JM, Rumessen JJ. Interstitial cells of Cajal in human gut and gastrointestinal disease. Microsc Res Tech. 1999;47:344–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

