Keratin mutation predisposes to mouse liver fibrosis and unmasks differential effects of the carbon tetrachloride and thioacetamide models
- PMID: 18395095
- PMCID: PMC2692280
- DOI: 10.1053/j.gastro.2008.01.035
Keratin mutation predisposes to mouse liver fibrosis and unmasks differential effects of the carbon tetrachloride and thioacetamide models
Abstract
Background & aims: Keratins 8 and 18 (K8/K18) are important hepatoprotective proteins. Animals expressing K8/K18 mutants show a marked susceptibility to acute/subacute liver injury. K8/K18 variants predispose to human end-stage liver disease and associate with fibrosis progression during chronic hepatitis C infection. We sought direct evidence for a keratin mutation-related predisposition to liver fibrosis using transgenic mouse models because the relationship between keratin mutations and cirrhosis is based primarily on human association studies.
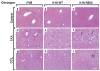
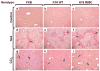
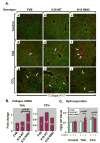
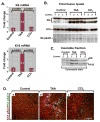
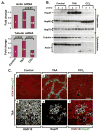
Methods: Mouse hepatofibrosis was induced by carbon tetrachloride (CCl(4)) or thioacetamide. Nontransgenic mice, or mice that over express either human Arg89-to-Cys (R89C mice) or wild-type K18 (WT mice) were used. The extent of fibrosis was evaluated by quantitative real-time reverse-transcription polymerase chain reaction of fibrosis-related genes, liver hydroxyproline measurement, and Picro-Sirius red staining and collagen immunofluorescence staining.
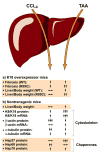
Results: Compared with control animals, CCl(4) led to similar liver fibrosis but increased injury in K18 R89C mice. In contrast, thioacetamide caused more severe liver injury and fibrosis in K18 R89C as compared with WT and nontransgenic mice and resulted in increased messenger RNA levels of collagen, tissue inhibitor of metalloproteinase 1, matrix metalloproteinase 2, and matrix metalloproteinase 13. Analysis in nontransgenic mice showed that thioacetamide and CCl(4) have dramatically different molecular expression responses involving cytoskeletal and chaperone proteins.
Conclusions: Over expression of K18 R89C predisposes transgenic mice to thioacetamide- but not CCl(4)-induced liver fibrosis. Differences in the keratin mutation-associated fibrosis response among the 2 models raise the hypothesis that keratin variants may preferentially predispose to fibrosis in unique human liver diseases. Findings herein highlight distinct differences in the 2 widely used fibrosis models.
Conflict of interest statement
Figures
Similar articles
-
Keratin mutation in transgenic mice predisposes to Fas but not TNF-induced apoptosis and massive liver injury.Hepatology. 2003 May;37(5):1006-14. doi: 10.1053/jhep.2003.50181. Hepatology. 2003. PMID: 12717381
-
Keratin overexpression levels correlate with the extent of spontaneous pancreatic injury.Am J Pathol. 2008 Apr;172(4):882-92. doi: 10.2353/ajpath.2008.070830. Epub 2008 Mar 18. Am J Pathol. 2008. PMID: 18349119 Free PMC article.
-
Keratins let liver live: Mutations predispose to liver disease and crosslinking generates Mallory-Denk bodies.Hepatology. 2007 Nov;46(5):1639-49. doi: 10.1002/hep.21976. Hepatology. 2007. PMID: 17969036 Review.
-
Keratin 18 overexpression but not phosphorylation or filament organization blocks mouse Mallory body formation.Hepatology. 2007 Jan;45(1):88-96. doi: 10.1002/hep.21471. Hepatology. 2007. PMID: 17187412
-
Keratins: markers and modulators of liver disease.Curr Opin Gastroenterol. 2012 May;28(3):209-16. doi: 10.1097/MOG.0b013e3283525cb8. Curr Opin Gastroenterol. 2012. PMID: 22450891 Review.
Cited by
-
Hepatocyte-Specific Deletion of Mouse Lamin A/C Leads to Male-Selective Steatohepatitis.Cell Mol Gastroenterol Hepatol. 2017 Jul 6;4(3):365-383. doi: 10.1016/j.jcmgh.2017.06.005. eCollection 2017 Nov. Cell Mol Gastroenterol Hepatol. 2017. PMID: 28913408 Free PMC article.
-
SNS-032 attenuates liver fibrosis by anti-active hepatic stellate cells via inhibition of cyclin dependent kinase 9.Front Pharmacol. 2022 Oct 12;13:1016552. doi: 10.3389/fphar.2022.1016552. eCollection 2022. Front Pharmacol. 2022. PMID: 36313366 Free PMC article.
-
The role of keratins in the digestive system: lessons from transgenic mouse models.Histochem Cell Biol. 2018 Oct;150(4):351-359. doi: 10.1007/s00418-018-1695-4. Epub 2018 Jul 24. Histochem Cell Biol. 2018. PMID: 30039330 Review.
-
Intermediate filaments take the heat as stress proteins.Trends Cell Biol. 2010 Feb;20(2):79-91. doi: 10.1016/j.tcb.2009.11.004. Epub 2010 Jan 4. Trends Cell Biol. 2010. PMID: 20045331 Free PMC article. Review.
-
A mouse model of cholestasis-associated cholangiocarcinoma and transcription factors involved in progression.Gastroenterology. 2011 Jul;141(1):378-88, 388.e1-4. doi: 10.1053/j.gastro.2011.03.044. Epub 2011 Mar 24. Gastroenterology. 2011. PMID: 21440549 Free PMC article.
References
-
- Ku N-O, Zhou X, Toivola DM, et al. The cytoskeleton of digestive epithelia in health and disease. Am J Physiol. 1999;277:G1108–1137. - PubMed
-
- Omary MB, Coulombe PA, McLean WH. Intermediate filament proteins and their associated diseases. N Engl J Med. 2004;351:2087–2100. - PubMed
-
- Herrmann H, Hesse M, Reichenzeller M, et al. Functional complexity of intermediate filament cytoskeletons: from structure to assembly to gene ablation. Int Rev Cytol. 2003;223:83–175. - PubMed
-
- Coulombe PA, Wong P. Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nat Cell Biol. 2004;6:699–706. - PubMed
-
- Worman HJ, Courvalin JC. Nuclear envelope, nuclear lamina, and inherited disease. Int Rev Cytol. 2005;246:231–279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

