The role of cerium redox state in the SOD mimetic activity of nanoceria
- PMID: 18395249
- PMCID: PMC2396488
- DOI: 10.1016/j.biomaterials.2008.03.014
The role of cerium redox state in the SOD mimetic activity of nanoceria
Abstract
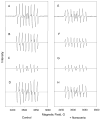
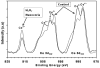
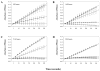
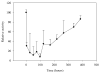
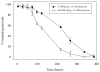
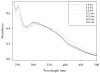
Cerium oxide nanoparticles (nanoceria) have recently been shown to protect cells against oxidative stress in both cell culture and animal models. Nanoceria has been shown to exhibit superoxide dismutase (SOD) activity using a ferricytochrome C assay, and this mimetic activity that has been postulated to be responsible for cellular protection by nanoceria. The nature of nanoceria's antioxidant properties, specifically what physical characteristics make nanoceria effective at scavenging superoxide anion, is poorly understood. In this study electron paramagnetic resonance (EPR) analysis confirms the reactivity of nanoceria as an SOD mimetic. X-ray photoelectron spectroscopy (XPS) and UV-visible analyses of nanoceria treated with hydrogen peroxide demonstrate that a decrease in the Ce 3(+)/4(+) ratio correlates directly with a loss of SOD mimetic activity. These results strongly suggest that the surface oxidation state of nanoceria plays an integral role in the SOD mimetic activity of nanoceria and that ability of nanoceria to scavenge superoxide is directly related to cerium(III) concentrations at the surface of the particle.
Figures

References
-
- Hu ZY, Haneklaus S, Sparovek G, Schnug E. Rare earth elements in soils. Commun Soil Sci Plan. 2006;37(9–10):1381–1420.
-
- Medalia A, Byrne B. Spectrophotometric Determination of of Cerium (IV) Anal Chem. 1951;23(3):453–456.
-
- Teeguarden JG, Hinderliter PM, Orr G, Thrall BD, Pounds JG. Particokinetics in vitro: dosimetry considerations for in vitro nanoparticle toxicity assessments. Toxicol Sci. 2007 Feb;95(2):300–312. - PubMed
-
- Esch F, Fabris S, Zhou L, Montini T, Africh C, Fornasiero P, et al. Electron localization determines defect formation on ceria substrates. Science. 2005 Jul 29;309(5735):752–755. - PubMed
-
- Campbell CT, Peden CH. Chemistry. Oxygen vacancies and catalysis on ceria surfaces. Science. 2005 Jul 29;309(5735):713–714. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

