Functions of RANKL/RANK/OPG in bone modeling and remodeling
- PMID: 18395508
- PMCID: PMC2413418
- DOI: 10.1016/j.abb.2008.03.018
Functions of RANKL/RANK/OPG in bone modeling and remodeling
Abstract
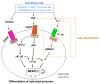
The discovery of the RANKL/RANK/OPG system in the mid 1990s for the regulation of bone resorption has led to major advances in our understanding of how bone modeling and remodeling are regulated. It had been known for many years before this discovery that osteoblastic stromal cells regulated osteoclast formation, but it had not been anticipated that they would do this through expression of members of the TNF superfamily: receptor activator of NF-kappaB ligand (RANKL) and osteoprotegerin (OPG), or that these cytokines and signaling through receptor activator of NF-kappaB (RANK) would have extensive functions beyond regulation of bone remodeling. RANKL/RANK signaling regulates osteoclast formation, activation and survival in normal bone modeling and remodeling and in a variety of pathologic conditions characterized by increased bone turnover. OPG protects bone from excessive resorption by binding to RANKL and preventing it from binding to RANK. Thus, the relative concentration of RANKL and OPG in bone is a major determinant of bone mass and strength. Here, we review our current understanding of the role of the RANKL/RANK/OPG system in bone modeling and remodeling.
Figures
References
-
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. - PubMed
-
- Del Fattore A, Cappariello A, Teti A. Genetics, pathogenesis and complications of osteopetrosis. Bone. 2008;42:19–29. - PubMed
-
- Tolar J, Teitelbaum SL, Orchard PJ. Osteopetrosis. N Engl J Med. 2004;351:2839–2849. - PubMed
-
- Lian JB, Stein GS. Runx2/Cbfa1: a multifunctional regulator of bone formation. Curr Pharm Des. 2003;9:2677–2685. - PubMed
-
- Karsenty G. Role of Cbfa1 in osteoblast differentiation and function. Semin Cell Dev Biol. 2000;11:343–346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

