TLR4 and S1P receptors cooperate to enhance inflammatory cytokine production in human gingival epithelial cells
- PMID: 18395849
- PMCID: PMC2738989
- DOI: 10.1002/eji.200737898
TLR4 and S1P receptors cooperate to enhance inflammatory cytokine production in human gingival epithelial cells
Abstract
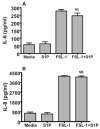
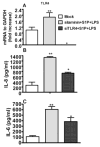
Toll-like receptors (TLR) are pattern recognition receptors for highly conserved microbial molecular patterns. Activation of TLR is a pivotal step in the initiation of innate, inflammatory, and immune defense mechanisms. Recent findings indicate that G protein-coupled receptors (GPCR) may modulate TLR signaling, but it is unclear which GPCR are involved in this process. One such cooperation between GPCR and TLR can be attributed to the sphingosine 1-phosphate (S1P) receptor family. The S1P receptors (S1P1-5) are a family of GPCR with a high affinity for S1P, a serum-borne bioactive lipid associated with diverse biological activities such as inflammation and healing. In this study, we show that pro-inflammatory cytokine production, including IL-6 and IL-8, was increased with LPS and concomitant S1P stimulation. Furthermore, elevated cytokine production following LPS and S1P challenge in human gingival epithelial cells (HGEC) was significantly reduced when TLR4, S1P1 or S1P3 signaling was blocked. Our study also shows that S1P1 and S1P3 expression was induced by LPS in HGEC, and this elevated expression enhanced the influence of S1P in its cooperation with TLR4 to increase cytokine production. This cooperation between TLR4 and S1P1 or S1P3 demonstrates that TLR4 and GPCR can interact to enhance cytokine production in epithelial cells.
Conflict of interest statement
Figures

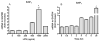

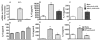
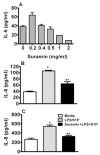
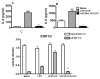
References
-
- Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, Nespoli A, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–514. - PubMed
-
- Kawai T, Akira S. Pathogen recognition with Toll-like receptors. Curr Opin Immunol. 2005;17:338–344. - PubMed
-
- Kaisho T, Akira S. Critical roles of Toll-like receptors in host defense. Crit Rev Immunol. 2000;20:393–405. - PubMed
-
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the DrosophilaToll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. - PubMed
-
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

