TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis
- PMID: 18396105
- PMCID: PMC3546119
- DOI: 10.1016/S1474-4422(08)70071-1
TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis
Abstract
Background: TDP-43 is a major component of the ubiquitinated inclusions that characterise amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) with ubiquitin inclusions (FTLD-U). TDP-43 is an RNA-binding and DNA-binding protein that has many functions and is encoded by the TAR DNA-binding protein gene (TARDBP) on chromosome 1. Our aim was to investigate whether TARDBP is a candidate disease gene for familial ALS that is not associated with mutations in superoxide dismutase 1 (SOD1).
Methods: TARDBP was sequenced in 259 patients with ALS, FTLD, or both. We used TaqMan-based SNP genotyping to screen for the identified variants in control groups matched to two kindreds of patients for age and ethnic origin. Additional clinical, genetic, and pathological assessments were made in these two families.
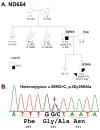
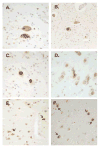
Findings: We identified two variants in TARDBP, which would encode Gly290Ala and Gly298Ser forms of TDP-43, in two kindreds with familial ALS. The variants seem to be pathogenic because they co-segregated with disease in both families, were absent in controls, and were associated with TDP-43 neuropathology in both members of one of these families for whom CNS tissue was available.
Interpretation: The Gly290Ala and Gly298Ser mutations are located in the glycine-rich domain of TDP-43, which regulates gene expression and mediates protein-protein interactions such as those with heterogeneous ribonucleoproteins. Owing to the varied and important cellular functions of TDP-43, these mutations might cause neurodegeneration through both gains and losses of function. The finding of pathogenic mutations in TARDBP implicates TDP-43 as an active mediator of neurodegeneration in TDP-43 proteinopathies, a class of disorder that includes ALS and FTLD-U.
Funding: National Institutes of Health (AG10124, AG17586, AG005136-22, PO1 AG14382), Department of Veterans Affairs, Friedrich-Baur Stiftung (0017/2007), US Public Health Service, ALS Association, and Fundació 'la Caixa'.
Figures


Similar articles
-
ALS and FTLD: two faces of TDP-43 proteinopathy.Eur J Neurol. 2008 Aug;15(8):772-80. doi: 10.1111/j.1468-1331.2008.02195.x. Eur J Neurol. 2008. PMID: 18684309 Free PMC article. Review.
-
Amyotrophic lateral sclerosis-frontotemporal lobar dementia in 3 families with p.Ala382Thr TARDBP mutations.Arch Neurol. 2010 Aug;67(8):1002-9. doi: 10.1001/archneurol.2010.173. Arch Neurol. 2010. PMID: 20697052 Free PMC article.
-
Novel mutations in TARDBP (TDP-43) in patients with familial amyotrophic lateral sclerosis.PLoS Genet. 2008 Sep 19;4(9):e1000193. doi: 10.1371/journal.pgen.1000193. PLoS Genet. 2008. PMID: 18802454 Free PMC article.
-
[TDP-43 proteinopathies: ALS and frontotemporal dementias].Fortschr Neurol Psychiatr. 2009 Aug;77 Suppl 1:S25-7. doi: 10.1055/s-0028-1109602. Epub 2009 Aug 14. Fortschr Neurol Psychiatr. 2009. PMID: 19685386 German.
-
Molecular neuropathology of TDP-43 proteinopathies.Int J Mol Sci. 2009 Jan;10(1):232-246. doi: 10.3390/ijms10010232. Epub 2009 Jan 9. Int J Mol Sci. 2009. PMID: 19333444 Free PMC article. Review.
Cited by
-
Increase in hnRNPA1 Expression Suffices to Kill Motor Neurons in Transgenic Rats.Int J Mol Sci. 2023 Nov 11;24(22):16214. doi: 10.3390/ijms242216214. Int J Mol Sci. 2023. PMID: 38003404 Free PMC article.
-
Dysregulation of RNA-Binding Proteins in Amyotrophic Lateral Sclerosis.Front Mol Neurosci. 2020 May 29;13:78. doi: 10.3389/fnmol.2020.00078. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32547363 Free PMC article.
-
The complex molecular biology of amyotrophic lateral sclerosis (ALS).Prog Mol Biol Transl Sci. 2012;107:215-62. doi: 10.1016/B978-0-12-385883-2.00002-3. Prog Mol Biol Transl Sci. 2012. PMID: 22482452 Free PMC article. Review.
-
Where and Why Modeling Amyotrophic Lateral Sclerosis.Int J Mol Sci. 2021 Apr 12;22(8):3977. doi: 10.3390/ijms22083977. Int J Mol Sci. 2021. PMID: 33921446 Free PMC article. Review.
-
The Potential Connection between Molecular Changes and Biomarkers Related to ALS and the Development and Regeneration of CNS.Int J Mol Sci. 2022 Sep 26;23(19):11360. doi: 10.3390/ijms231911360. Int J Mol Sci. 2022. PMID: 36232667 Free PMC article. Review.
References
-
- Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52(1):39–59. - PubMed
-
- Gros-Louis F, Gaspar C, Rouleau GA. Genetics of familial and sporadic amyotrophic lateral sclerosis. Biochim Biophys Acta. 2006;1762(11-12):956–72. - PubMed
-
- Hadano S, Hand CK, Osuga H, et al. A gene encoding a putative GTPase regulator is mutated in familial amyotrophic lateral sclerosis 2. Nat Genet. 2001;29(2):166–73. - PubMed
-
- Yang Y, Hentati A, Deng HX, et al. The gene encoding alsin, a protein with three guanine-nucleotide exchange factor domains, is mutated in a form of recessive amyotrophic lateral sclerosis. Nat Genet. 2001;29(2):160–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

