SARS vaccine based on a replication-defective recombinant vesicular stomatitis virus is more potent than one based on a replication-competent vector
- PMID: 18396306
- PMCID: PMC7103385
- DOI: 10.1016/j.virol.2008.03.002
SARS vaccine based on a replication-defective recombinant vesicular stomatitis virus is more potent than one based on a replication-competent vector
Abstract
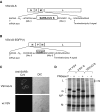
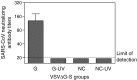
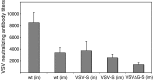
A SARS vaccine based on a live-attenuated vesicular stomatitis virus (VSV) recombinant expressing the SARS-CoV S protein provides long-term protection of immunized mice from SARS-CoV infection (Kapadia, S.U., Rose, J. K., Lamirande, E., Vogel, L., Subbarao, K., Roberts, A., 2005. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology 340(2), 174-82.). Because it is difficult to obtain regulatory approval of vaccine based on live viruses, we constructed a replication-defective single-cycle VSV vector in which we replaced the VSV glycoprotein (G) gene with the SARS-CoV S gene. The virus was only able to infect cells when pseudotyped with the VSV G protein. We measured the effectiveness of immunization with the single-cycle vaccine in mice. We found that the vaccine given intramuscularly induced a neutralizing antibody response to SARS-CoV that was approximately ten-fold greater than that required for the protection from SARS-CoV infection, and significantly greater than that generated by the replication-competent vector expressing SARS-CoV S protein given by the same route. Our results, along with earlier studies showing potent induction of T-cell responses by single-cycle vectors, indicate that these vectors are excellent alternatives to live-attenuated VSV.
Figures

Similar articles
-
Replication-Competent Vesicular Stomatitis Virus Vaccine Vector Protects against SARS-CoV-2-Mediated Pathogenesis in Mice.Cell Host Microbe. 2020 Sep 9;28(3):465-474.e4. doi: 10.1016/j.chom.2020.07.018. Epub 2020 Jul 30. Cell Host Microbe. 2020. PMID: 32798445 Free PMC article.
-
A single immunization with a rhabdovirus-based vector expressing severe acute respiratory syndrome coronavirus (SARS-CoV) S protein results in the production of high levels of SARS-CoV-neutralizing antibodies.J Gen Virol. 2005 May;86(Pt 5):1435-1440. doi: 10.1099/vir.0.80844-0. J Gen Virol. 2005. PMID: 15831955 Free PMC article.
-
Recombinant modified vaccinia virus Ankara expressing the spike glycoprotein of severe acute respiratory syndrome coronavirus induces protective neutralizing antibodies primarily targeting the receptor binding region.J Virol. 2005 Mar;79(5):2678-88. doi: 10.1128/JVI.79.5.2678-2688.2005. J Virol. 2005. PMID: 15708987 Free PMC article.
-
Vaccine design for severe acute respiratory syndrome coronavirus.Viral Immunol. 2005;18(2):327-32. doi: 10.1089/vim.2005.18.327. Viral Immunol. 2005. PMID: 16035944 Review.
-
Pseudotyped Vesicular Stomatitis Virus-Severe Acute Respiratory Syndrome-Coronavirus-2 Spike for the Study of Variants, Vaccines, and Therapeutics Against Coronavirus Disease 2019.Front Microbiol. 2022 Jan 14;12:817200. doi: 10.3389/fmicb.2021.817200. eCollection 2021. Front Microbiol. 2022. PMID: 35095820 Free PMC article. Review.
Cited by
-
A viable recombinant rhabdovirus lacking its glycoprotein gene and expressing influenza virus hemagglutinin and neuraminidase is a potent influenza vaccine.J Virol. 2015 Mar;89(5):2820-30. doi: 10.1128/JVI.03246-14. Epub 2014 Dec 24. J Virol. 2015. PMID: 25540378 Free PMC article.
-
Replication-Competent Vesicular Stomatitis Virus Vaccine Vector Protects against SARS-CoV-2-Mediated Pathogenesis in Mice.Cell Host Microbe. 2020 Sep 9;28(3):465-474.e4. doi: 10.1016/j.chom.2020.07.018. Epub 2020 Jul 30. Cell Host Microbe. 2020. PMID: 32798445 Free PMC article.
-
Replication-competent vesicular stomatitis virus vaccine vector protects against SARS-CoV-2-mediated pathogenesis.bioRxiv [Preprint]. 2020 Jul 10:2020.07.09.196386. doi: 10.1101/2020.07.09.196386. bioRxiv. 2020. Update in: Cell Host Microbe. 2020 Sep 9;28(3):465-474.e4. doi: 10.1016/j.chom.2020.07.018. PMID: 32676597 Free PMC article. Updated. Preprint.
-
Refined methods for propagating vesicular stomatitis virus vectors that are defective for G protein expression.J Virol Methods. 2010 Mar;164(1-2):43-50. doi: 10.1016/j.jviromet.2009.11.023. Epub 2009 Nov 24. J Virol Methods. 2010. PMID: 19941901 Free PMC article.
-
Plant Viruses in Plant Molecular Pharming: Toward the Use of Enveloped Viruses.Front Plant Sci. 2019 Jun 19;10:803. doi: 10.3389/fpls.2019.00803. eCollection 2019. Front Plant Sci. 2019. PMID: 31275344 Free PMC article. Review.
References
-
- Daddario-DiCaprio K.M., Geisbert T.W., Stroher U., Geisbert J.B., Grolla A., Fritz E.A., Fernando L., Kagan E., Jahrling P.B., Hensley L.E., Jones S.M., Feldmann H. Postexposure protection against Marburg haemorrhagic fever with recombinant vesicular stomatitis virus vectors in non-human primates: an efficacy assessment. Lancet. 2006;367(9520):1399–1404. - PubMed
-
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1967–1976. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

