Cell proliferation, apoptosis and mitochondrial damage in rat B50 neuronal cells after cisplatin treatment
- PMID: 18397337
- PMCID: PMC6496376
- DOI: 10.1111/j.1365-2184.2008.00530.x
Cell proliferation, apoptosis and mitochondrial damage in rat B50 neuronal cells after cisplatin treatment
Abstract
Objectives: Cisplatin (cisPt) is used as a chemotherapeutic agent for the treatment of a variety of human tumours; more recently, it has been demonstrated that tumour cell exposure to cisPt ultimately results in apoptosis, but the mechanism by which nuclear cisPt/DNA generates the cytoplasmic cascade of events involved has not been clarified. We have investigated the effects of cisPt on proliferation in the neuronal cell line B50, with particular attention being given to understand whether mitochondria are a target of cisPt and their involvement in the apoptotic process.
Materials and methods: Rat neuronal B50 cells were used to investigate the mechanisms of cisPt-induced cytotoxicity; this line has been used as a model system for neurotoxicity in vivo.
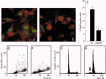
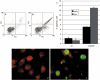
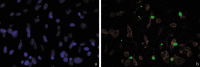
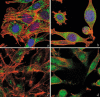
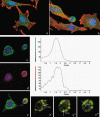
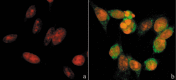
Results: Changes in proliferation, induction of apoptosis, activation of caspase-3 and DNA fragmentation were observed in the cells, as well as morphological and biochemical alterations of mithocondria. Activation of caspase-9 confirmed that mitochondria are a target of cisPt.
Conclusion: CisPt exerts cytotoxic effects in the neuronal B50 cell line via a caspase-dependent pathway with mitochondria being central relay stations.
Figures

References
-
- Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale F, Sadoul R, Martinou JC (1997) Inhibition of Bax channel‐forming activity by Bcl‐2. Science 277, 370–372. - PubMed
-
- Antonsson B, Montessuit S, Sanchez B, Martinou JC (2001) Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 276, 11615–11623. - PubMed
-
- Avella D, Pisu MB, Roda E, Gravati M, Bernocchi G (2006) Reorganization of the rat cerebellar cortex during postnatal development following cisplatin treatment. Exp. Neurol. 201, 131–143. - PubMed
-
- Bedner E, Li X, Kunicki J, Darzynkiewicz Z (2000) Translocation of Bax to mitochondria during apoptosis measured by laser scanning cytometry. Cytometry 41, 83–88. - PubMed
-
- Bernocchi G, Scherini E, Nano R (1990) Developmental patterns in the rat cerebellum after cis‐dichlorodiammineplatinum treatment. Neuroscience 39, 179–188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

