Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells
- PMID: 18397755
- PMCID: PMC3717373
- DOI: 10.1016/j.stem.2008.02.011
Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells
Abstract
Gain-of-function experiments have demonstrated the potential of Notch signals to expand primitive hematopoietic progenitors, but whether Notch physiologically regulates hematopoietic stem cell (HSC) homeostasis in vivo is unclear. To answer this question, we evaluated the effect of global deficiencies of canonical Notch signaling in rigorous HSC assays. Hematopoietic progenitors expressing dominant-negative Mastermind-like1 (DNMAML), a potent inhibitor of Notch-mediated transcriptional activation, achieved stable long-term reconstitution of irradiated hosts and showed a normal frequency of progenitor fractions enriched for long-term HSCs. Similar results were observed with cells lacking CSL/RBPJ, a DNA-binding factor that is required for canonical Notch signaling. Notch-deprived progenitors provided normal long-term reconstitution after secondary competitive transplantation. Furthermore, Notch target genes were expressed at low levels in primitive hematopoietic progenitors. Taken together, these results rule out an essential physiological role for cell-autonomous canonical Notch signals in HSC maintenance.
Figures




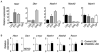
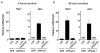
Comment in
-
Notch in the niche.Cell Stem Cell. 2008 Apr 10;2(4):293-4. doi: 10.1016/j.stem.2008.03.016. Cell Stem Cell. 2008. PMID: 18397744
References
-
- Adolfsson J, Borge OJ, Bryder D, Theilgaard-Monch K, Astrand-Grundstrom I, Sitnicka E, Sasaki Y, Jacobsen SE. Upregulation of Flt3 expression within the bone marrow Lin(−)Sca1(+)c- kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. - PubMed
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
-
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the hematopoietic stem cell niche. Nature. 2003;425:841–846. - PubMed
-
- Carlesso N, Aster JC, Sklar J, Scadden DT. Notch1-induced delay of human hematopoietic progenitor cell differentiation is associated with altered cell cycle kinetics. Blood. 1999;93:838–848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

