Identification and regulation of TPS04/GES, an Arabidopsis geranyllinalool synthase catalyzing the first step in the formation of the insect-induced volatile C16-homoterpene TMTT
- PMID: 18398052
- PMCID: PMC2390743
- DOI: 10.1105/tpc.106.049478
Identification and regulation of TPS04/GES, an Arabidopsis geranyllinalool synthase catalyzing the first step in the formation of the insect-induced volatile C16-homoterpene TMTT
Abstract
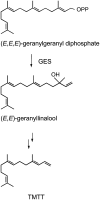
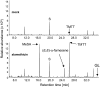
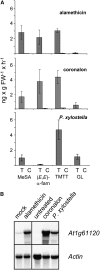
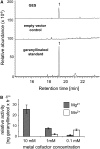
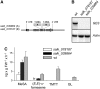
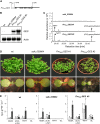
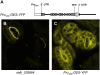
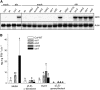
Volatile secondary metabolites emitted by plants contribute to plant-plant, plant-fungus, and plant-insect interactions. The C(16)-homoterpene TMTT (for 4,8,12-trimethyltrideca-1,3,7,11-tetraene) is emitted after herbivore attack by a wide variety of plant species, including Arabidopsis thaliana, and is assumed to play a role in attracting predators or parasitoids of herbivores. TMTT has been suggested to be formed as a degradation product of the diterpene alcohol (E,E)-geranyllinalool. Here, we report the identification of Terpene Synthase 04 (TPS04; At1g61120) as a geranyllinalool synthase (GES). Recombinant TPS04/GES protein expressed in Escherichia coli catalyzes the formation of (E,E)-geranyllinalool from the substrate geranylgeranyl diphosphate. Transgenic Arabidopsis lines carrying T-DNA insertions in the TPS04 locus are deficient in (E,E)-geranyllinalool and TMTT synthesis, a phenotype that can be complemented by expressing the GES gene under the control of a heterologous promoter. GES transcription is upregulated under conditions that induce (E,E)-geranyllinalool and TMTT synthesis, including infestation of plants with larvae of the moth Plutella xylostella and treatment with the fungal peptide alamethicin or the octadecanoid mimic coronalon. Induction requires jasmonic acid but is independent from salicylic acid or ethylene. This study paves the ground to address the contribution of TMTT in ecological interactions and to elucidate the signaling network that regulates TMTT synthesis.
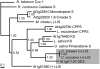
Figures


References
-
- Ament, K., Van Schie, C.C., Bouwmeester, H.J., Haring, M.A., and Schuurink, R.C. (2006). Induction of a leaf specific geranylgeranyl pyrophosphate synthase and emission of (E,E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene in tomato are dependent on both jasmonic acid and salicylic acid signaling pathways. Planta 224 1197–1208. - PubMed
-
- An, Y.Q., McDowell, J.M., Huang, S., McKinney, E.C., Chambliss, S., and Meagher, R.B. (1996). Strong, constitutive expression of the Arabidopsis ACT2/ACT8 actin subclass in vegetative tissues. Plant J. 10 107–121. - PubMed
-
- Arimura, G., Ozawa, R., Shimoda, T., Nishioka, T., Boland, W., and Takabayashi, J. (2000). Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 406 512–515. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

