A randomized placebo-controlled trial of intermittent preventive treatment in pregnant women in the context of insecticide treated nets delivered through the antenatal clinic
- PMID: 18398460
- PMCID: PMC2277457
- DOI: 10.1371/journal.pone.0001934
A randomized placebo-controlled trial of intermittent preventive treatment in pregnant women in the context of insecticide treated nets delivered through the antenatal clinic
Abstract
Background: Current recommendations to prevent malaria in African pregnant women rely on insecticide treated nets (ITNs) and intermittent preventive treatment (IPTp). However, there is no information on the safety and efficacy of their combined use.
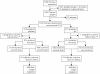
Methods: 1030 pregnant Mozambican women of all gravidities received a long-lasting ITN during antenatal clinic (ANC) visits and, irrespective of HIV status, were enrolled in a randomised, double blind, placebo-controlled trial, to assess the safety and efficacy of 2-dose sulphadoxine-pyrimethamine (SP). The main outcome was the reduction in low birth weight.
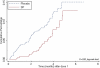
Findings: Two-dose SP was safe and well tolerated, but was not associated with reductions in anaemia prevalence at delivery (RR, 0.92 [95% CI, 0.79-1.08]), low birth weight (RR, 0.99 [95% CI, 0.70-1.39]), or overall placental infection (p = 0.964). However, the SP group showed a 40% reduction (95% CI, 7.40-61.20]; p = 0.020) in the incidence of clinical malaria during pregnancy, and reductions in the prevalence of peripheral parasitaemia (7.10% vs 15.15%) (p<0.001), and of actively infected placentas (7.04% vs 13.60%) (p = 0.002). There was a reduction in severe anaemia at delivery of borderline statistical significance (p = 0.055). These effects were not modified by gravidity or HIV status. Reported ITN's use was more than 90% in both groups.
Conclusions: Two-dose SP was associated with a reduction in some indicators, but these were not translated to significant improvement in other maternal or birth outcomes. The use of ITNs during pregnancy may reduce the need to administer IPTp. ITNs should be part of the ANC package in sub-Saharan Africa.
Trial registration: ClinicalTrials.gov NCT00209781.
Conflict of interest statement
Figures
References
-
- World Health Organization (WHO) Brazzaville, Democratic Republic of Congo: WHO Regional Office for Africa,2004; 2006. A strategic framework for malaria prevention and control during pregnancy in the African region: report AFR/MAL/04/01.
-
- Greenwood BM, Greenwood AM, Snow RW, Byass P, Bennett S, Hatibnjie AB. The Effects of Malaria Chemoprophylaxis Given by Traditional Birth Attendants on the Course and Outcome of Pregnancy. Trans R Soc Trop Med Hyg. 1989;83(5):589–94. - PubMed
-
- Brabin B. An Assessment of Low-Birth-Weight Risk in Primiparae As An Indicator of Malaria Control in Pregnancy. Int J Epi. 1991;20(1):276–83. - PubMed
-
- Menendez C, Ordi J, Ismail MR, Ventura PJ, Aponte JJ, Kahigwa E, et al. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181(5):1740–5. - PubMed
-
- Granja AC, Machungo F, Gomes A, Bergstrom S, Brabin B. Malaria-related maternal mortality in urban Mozambique. Ann Trop Med Parasitol. 1998;92(3):257–63. - PubMed

