Glial progenitor-like phenotype in low-grade glioma and enhanced CD133-expression and neuronal lineage differentiation potential in high-grade glioma
- PMID: 18398462
- PMCID: PMC2277459
- DOI: 10.1371/journal.pone.0001936
Glial progenitor-like phenotype in low-grade glioma and enhanced CD133-expression and neuronal lineage differentiation potential in high-grade glioma
Abstract
Background: While neurosphere- as well as xenograft tumor-initiating cells have been identified in gliomas, the resemblance between glioma cells and neural stem/progenitor cells as well as the prognostic value of stem/progenitor cell marker expression in glioma are poorly clarified.
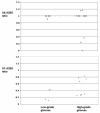
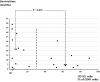
Methodology/principal findings: Viable glioma cells were characterized for surface marker expression along the glial genesis hierarchy. Six low-grade and 17 high-grade glioma specimens were flow-cytometrically analyzed for markers characteristics of stem cells (CD133); glial progenitors (PDGFRalpha, A2B5, O4, and CD44); and late oligodendrocyte progenitors (O1). In parallel, the expression of glial fibrillary acidic protein (GFAP), synaptophysin and neuron-specific enolase (NSE) was immunohistochemically analyzed in fixed tissue specimens. Irrespective of the grade and morphological diagnosis of gliomas, glioma cells concomitantly expressed PDGFRalpha, A2B5, O4, CD44 and GFAP. In contrast, O1 was weakly expressed in all low-grade and the majority of high-grade glioma specimens analyzed. Co-expression of neuronal markers was observed in all high-grade, but not low-grade, glioma specimens analyzed. The rare CD133 expressing cells in low-grade glioma specimens typically co-expressed vessel endothelial marker CD31. In contrast, distinct CD133 expression profiles in up to 90% of CD45-negative glioma cells were observed in 12 of the 17 high-grade glioma specimens and the majority of these CD133 expressing cells were CD31 negative. The CD133 expression correlates inversely with length of patient survival. Surprisingly, cytogenetic analysis showed that gliomas contained normal and abnormal cell karyotypes with hitherto indistinguishable phenotype.
Conclusions/significance: This study constitutes an important step towards clarification of lineage commitment and differentiation blockage of glioma cells. Our data suggest that glioma cells may resemble expansion of glial lineage progenitor cells with compromised differentiation capacity downstream of A2B5 and O4 expression. The concurrent expression of neuronal markers demonstrates that high-grade glioma cells are endowed with multi-lineage differentiation potential in vivo. Importantly, enhanced CD133 expression marks a poor prognosis in gliomas.
Conflict of interest statement
Figures




Similar articles
-
Identification of A2B5+CD133- tumor-initiating cells in adult human gliomas.Neurosurgery. 2008 Feb;62(2):505-14; discussion 514-5. doi: 10.1227/01.neu.0000316019.28421.95. Neurosurgery. 2008. PMID: 18382330
-
CD133 expression and cancer stem cells predict prognosis in high-grade oligodendroglial tumors.Brain Pathol. 2008 Jul;18(3):370-7. doi: 10.1111/j.1750-3639.2008.00130.x. Epub 2008 Mar 26. Brain Pathol. 2008. PMID: 18371181 Free PMC article.
-
Glioma cell populations grouped by different cell type markers drive brain tumor growth.Cancer Res. 2010 Jun 1;70(11):4274-9. doi: 10.1158/0008-5472.CAN-09-3904. Epub 2010 May 11. Cancer Res. 2010. PMID: 20460538
-
What is the clinical value of cancer stem cell markers in gliomas?Int J Clin Exp Pathol. 2013;6(3):334-48. Epub 2013 Feb 15. Int J Clin Exp Pathol. 2013. PMID: 23412423 Free PMC article. Review.
-
[Glial stem cells and their relationship with tumour angiogenesis process].Rev Neurol. 2011 Jun 16;52(12):743-50. Rev Neurol. 2011. PMID: 21594859 Review. Spanish.
Cited by
-
PTEN status is related to cell proliferation and self-renewal independent of CD133 phenotype in the glioma-initiating cells.Mol Cell Biochem. 2011 Mar;349(1-2):149-57. doi: 10.1007/s11010-010-0669-1. Epub 2010 Nov 26. Mol Cell Biochem. 2011. PMID: 21110069
-
A non-aggressive, highly efficient, enzymatic method for dissociation of human brain-tumors and brain-tissues to viable single-cells.BMC Neurosci. 2016 Jun 1;17(1):30. doi: 10.1186/s12868-016-0262-y. BMC Neurosci. 2016. PMID: 27251756 Free PMC article.
-
Therapy targets in glioblastoma and cancer stem cells: lessons from haematopoietic neoplasms.J Cell Mol Med. 2013 Oct;17(10):1218-35. doi: 10.1111/jcmm.12122. Epub 2013 Sep 2. J Cell Mol Med. 2013. PMID: 23998913 Free PMC article. Review.
-
Progranulin promotes Temozolomide resistance of glioblastoma by orchestrating DNA repair and tumor stemness.Oncogene. 2015 Apr 2;34(14):1853-64. doi: 10.1038/onc.2014.92. Epub 2014 May 5. Oncogene. 2015. PMID: 24793792
-
TLX-Its Emerging Role for Neurogenesis in Health and Disease.Mol Neurobiol. 2017 Jan;54(1):272-280. doi: 10.1007/s12035-015-9608-1. Epub 2016 Jan 6. Mol Neurobiol. 2017. PMID: 26738856 Free PMC article. Review.
References
-
- Bouvier C, Bartoli C, Aguirre-Cruz L, Virard I, Colin C, et al. Shared oligodendrocyte lineage gene expression in gliomas and oligodendrocyte progenitor cells. J Neurosurg. 2003;99:344–50. - PubMed
-
- Ligon KL, Alberta JA, Kho AT, Weiss J, Kwaan MR, et al. The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J Neuropathol Exp Neurol. 2004;63:499–509. - PubMed
-
- Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, et al. CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

