The homeodomain derived peptide Penetratin induces curvature of fluid membrane domains
- PMID: 18398464
- PMCID: PMC2276244
- DOI: 10.1371/journal.pone.0001938
The homeodomain derived peptide Penetratin induces curvature of fluid membrane domains
Abstract
Background: Protein membrane transduction domains that are able to cross the plasma membrane are present in several transcription factors, such as the homeodomain proteins and the viral proteins such as Tat of HIV-1. Their discovery resulted in both new concepts on the cell communication during development, and the conception of cell penetrating peptide vectors for internalisation of active molecules into cells. A promising cell penetrating peptide is Penetratin, which crosses the cell membranes by a receptor and metabolic energy-independent mechanism. Recent works have claimed that Penetratin and similar peptides are internalized by endocytosis, but other endocytosis-independent mechanisms have been proposed. Endosomes or plasma membranes crossing mechanisms are not well understood. Previously, we have shown that basic peptides induce membrane invaginations suggesting a new mechanism for uptake, "physical endocytosis".
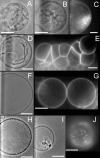
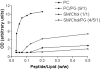
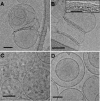
Methodology/principal findings: Herein, we investigate the role of membrane lipid phases on Penetratin induced membrane deformations (liquid ordered such as in "raft" microdomains versus disordered fluid "non-raft" domains) in membrane models. Experimental data show that zwitterionic lipid headgroups take part in the interaction with Penetratin suggesting that the external leaflet lipids of cells plasma membrane are competent for peptide interaction in the absence of net negative charges. NMR and X-ray diffraction data show that the membrane perturbations (tubulation and vesiculation) are associated with an increase in membrane negative curvature. These effects on curvature were observed in the liquid disordered but not in the liquid ordered (raft-like) membrane domains.
Conclusions/significance: The better understanding of the internalisation mechanisms of protein transduction domains will help both the understanding of the mechanisms of cell communication and the development of potential therapeutic molecular vectors. Here we showed that the membrane targets for these molecules are preferentially the fluid membrane domains and that the mechanism involves the induction of membrane negative curvature. Consequences on cellular uptake are discussed.
Conflict of interest statement
Figures

References
-
- Prochiantz A. Messenger proteins: homeoproteins, TAT and others. Curr Opin Cell Biol. 2000;12:400–406. - PubMed
-
- Prochiantz A, Joliot A. Can transcription factors function as cell-cell signalling molecules? Nat Rev Mol Cell Biol. 2003;4:814–819. - PubMed
-
- Mae M, Langel U. Cell-penetrating peptides as vectors for peptide, protein and oligonucleotide delivery. Curr Opin Pharmacol. 2006;6:509–514. - PubMed
-
- Murriel CL, Dowdy SF. Influence of protein transduction domains on intracellular delivery of macromolecules. Expert Opin Drug Deliv. 2006;3:739–746. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

