Safety and immunogenicity of a malaria vaccine, Plasmodium falciparum AMA-1/MSP-1 chimeric protein formulated in montanide ISA 720 in healthy adults
- PMID: 18398475
- PMCID: PMC2276862
- DOI: 10.1371/journal.pone.0001952
Safety and immunogenicity of a malaria vaccine, Plasmodium falciparum AMA-1/MSP-1 chimeric protein formulated in montanide ISA 720 in healthy adults
Abstract
Background: The P. falciparum chimeric protein 2.9 (PfCP-2.9) consisting of the sequences of MSP1-19 and AMA-1 (III) is a malaria vaccine candidate that was found to induce inhibitory antibodies in rabbits and monkeys. This was a phase I randomized, single-blind, placebo-controlled, dose-escalation study to evaluate the safety and immunogenicity of the PfCP-2.9 formulated with a novel adjuvant Montanide ISA720. Fifty-two subjects were randomly assigned to 4 dose groups of 10 participants, each receiving the test vaccine of 20, 50, 100, or 200 microg respectively, and 1 placebo group of 12 participants receiving the adjuvant only.
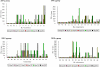
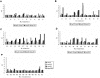
Methods and findings: The vaccine formulation was shown to be safe and well-tolerated, and none of the participants withdrew. The total incidence of local adverse events (AEs) was 75%, distributed among 58% of the placebo group and 80% of those vaccinated. Among the vaccinated, 65% had events that were mild and 15% experienced moderate AEs. Almost all systemic adverse reactions observed in this study were graded as mild and required no therapy. The participants receiving the test vaccine developed detectable antibody responses which were boosted by the repeated vaccinations. Sixty percent of the vaccinated participants had high ELISA titers (>1:10,000) of antigen-specific antibodies which could also recognize native parasite proteins in an immunofluorescence assay (IFA).
Conclusion: This study is the first clinical trial for this candidate and builds on previous investigations supporting PfCP-2.9/ISA720 as a promising blood-stage malaria vaccine. Results demonstrate safety, tolerability (particularly at the lower doses tested) and immunogenicity of the formulation. Further clinical development is ongoing to explore optimizing the dose and schedule of the formulation to decrease reactogenicity without compromising immunogenicity.
Trial registration: Chinese State Food and Drug Administration (SFDA) 2002SL0046; Controlled-Trials.com ISRCTN66850051 [66850051].
Conflict of interest statement
Figures

Similar articles
-
A phase 1 trial of MSP2-C1, a blood-stage malaria vaccine containing 2 isoforms of MSP2 formulated with Montanide® ISA 720.PLoS One. 2011;6(9):e24413. doi: 10.1371/journal.pone.0024413. Epub 2011 Sep 19. PLoS One. 2011. PMID: 21949716 Free PMC article. Clinical Trial.
-
Stability and potency of the Plasmodium falciparum MSP1-19/AMA-1(III) chimeric vaccine candidate with Montanide ISA720 adjuvant.Vaccine. 2010 Apr 19;28(18):3152-8. doi: 10.1016/j.vaccine.2010.02.054. Epub 2010 Mar 1. Vaccine. 2010. PMID: 20197139
-
Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51.PLoS One. 2008 Jul 9;3(7):e2636. doi: 10.1371/journal.pone.0002636. PLoS One. 2008. PMID: 18612426 Free PMC article. Clinical Trial.
-
Solution structure of a Plasmodium falciparum AMA-1/MSP 1 chimeric protein vaccine candidate (PfCP-2.9) for malaria.Malar J. 2010 Mar 18;9:76. doi: 10.1186/1475-2875-9-76. Malar J. 2010. PMID: 20236549 Free PMC article.
-
Towards an RTS,S-based, multi-stage, multi-antigen vaccine against falciparum malaria: progress at the Walter Reed Army Institute of Research.Vaccine. 2005 Mar 18;23(17-18):2243-50. doi: 10.1016/j.vaccine.2005.01.142. Vaccine. 2005. PMID: 15755604 Review.
Cited by
-
Phase 1 trial of the Plasmodium falciparum blood stage vaccine MSP1(42)-C1/Alhydrogel with and without CPG 7909 in malaria naïve adults.PLoS One. 2010 Jan 22;5(1):e8787. doi: 10.1371/journal.pone.0008787. PLoS One. 2010. PMID: 20107498 Free PMC article. Clinical Trial.
-
The use of proteomics for the identification of promising vaccine and diagnostic biomarkers in Plasmodium falciparum.Parasitology. 2020 Oct;147(12):1255-1262. doi: 10.1017/S003118202000102X. Epub 2020 Jun 19. Parasitology. 2020. PMID: 32618524 Free PMC article. Review.
-
Construction of transgenic Plasmodium berghei as a model for evaluation of blood-stage vaccine candidate of Plasmodium falciparum chimeric protein 2.9.PLoS One. 2009 Sep 3;4(9):e6894. doi: 10.1371/journal.pone.0006894. PLoS One. 2009. PMID: 19727400 Free PMC article.
-
A review of malaria vaccine clinical projects based on the WHO rainbow table.Malar J. 2012 Jan 9;11:11. doi: 10.1186/1475-2875-11-11. Malar J. 2012. PMID: 22230255 Free PMC article. Review.
-
Malaria vaccines and their potential role in the elimination of malaria.Malar J. 2008 Dec 11;7 Suppl 1(Suppl 1):S10. doi: 10.1186/1475-2875-7-S1-S10. Malar J. 2008. PMID: 19091034 Free PMC article. Review.
References
-
- Greenwood BM, Bojang K, Whitty CJ, Targett GA. Malaria. Lancet. 2005;365:1487–1498. - PubMed
-
- McGregor IA. The development and maintenance of immunity to malaria in highly endemic areas. Clinics Trop Med Commun Dis. 1986;1:29–53.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

