Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice
- PMID: 18398510
- PMCID: PMC2289794
- DOI: 10.1172/JCI33680
Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice
Erratum in
- J Clin Invest. 2008 Jun;118(6):2366
Abstract
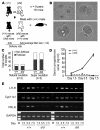
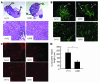
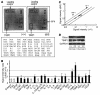
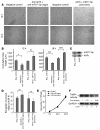
The microRNA (miRNA) processing enzyme Dicer1 is required for zygotic and embryonic development, but the early embryonic lethality of Dicer1 null alleles in mice has limited our ability to address the role of Dicer1 in normal mouse growth and development. To address this question, we used a mouse mutant with a hypomorphic Dicer1 allele (Dicer(d/d)) and found that Dicer1 deficiency resulted in female infertility. This defect in female Dicer(d/d) mice was caused by corpus luteum (CL) insufficiency and resulted, at least in part, from the impaired growth of new capillary vessels in the ovary. We found that the impaired CL angiogenesis in Dicer(d/d) mice was associated with a lack of miR17-5p and let7b, 2 miRNAs that participate in angiogenesis by regulating the expression of the antiangiogenic factor tissue inhibitor of metalloproteinase 1. Furthermore, injection of miR17-5p and let7b into the ovaries of Dicer(d/d) mice partially normalized tissue inhibitor of metalloproteinase 1 expression and CL angiogenesis. Our data indicate that the development and function of the ovarian CL is a physiological process that appears to be regulated by miRNAs and requires Dicer1 function.
Figures


Similar articles
-
The regulatory role of Dicer in folliculogenesis in mice.Mol Cell Endocrinol. 2010 Feb 5;315(1-2):63-73. doi: 10.1016/j.mce.2009.09.021. Epub 2009 Sep 30. Mol Cell Endocrinol. 2010. PMID: 19799966 Free PMC article.
-
Cancer-associated somatic DICER1 hotspot mutations cause defective miRNA processing and reverse-strand expression bias to predominantly mature 3p strands through loss of 5p strand cleavage.J Pathol. 2013 Feb;229(3):400-9. doi: 10.1002/path.4135. J Pathol. 2013. PMID: 23132766
-
Functional requirement of dicer1 and miR-17-5p in reactive astrocyte proliferation after spinal cord injury in the mouse.Glia. 2014 Dec;62(12):2044-60. doi: 10.1002/glia.22725. Epub 2014 Jul 18. Glia. 2014. PMID: 25043492
-
Regulatory role of microRNAs in ovarian function.Fertil Steril. 2014 Jun;101(6):1524-30. doi: 10.1016/j.fertnstert.2014.04.024. Fertil Steril. 2014. PMID: 24882616 Review.
-
miRNA processing and human cancer: DICER1 cuts the mustard.Sci Transl Med. 2011 Nov 30;3(111):111ps46. doi: 10.1126/scitranslmed.3002493. Sci Transl Med. 2011. PMID: 22133720 Review.
Cited by
-
microRNAs in gliomas: small regulators of a big problem.Neuromolecular Med. 2009;11(3):208-22. doi: 10.1007/s12017-009-8087-9. Neuromolecular Med. 2009. PMID: 19731102 Review.
-
New insights into ovarian function.Handb Exp Pharmacol. 2010;(198):3-27. doi: 10.1007/978-3-642-02062-9_1. Handb Exp Pharmacol. 2010. PMID: 20839083 Free PMC article. Review.
-
MicroRNAs in endothelial cell homeostasis and vascular disease.Curr Opin Hematol. 2018 May;25(3):227-236. doi: 10.1097/MOH.0000000000000424. Curr Opin Hematol. 2018. PMID: 29547400 Free PMC article. Review.
-
Non-coding RNA in Ovarian Development and Disease.Adv Exp Med Biol. 2016;886:79-93. doi: 10.1007/978-94-017-7417-8_5. Adv Exp Med Biol. 2016. PMID: 26659488 Free PMC article. Review.
-
Expression and localization of matrix metalloproteinases (MMP-2, -7, -9) and their tissue inhibitors (TIMP-2, -3) in the chicken oviduct during maturation.Cell Tissue Res. 2016 Apr;364(1):185-97. doi: 10.1007/s00441-015-2290-9. Epub 2015 Sep 22. Cell Tissue Res. 2016. PMID: 26395636 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

