A novel phenotypic method to determine fludarabine triphosphate accumulation in T-lymphocytes from hematopoietic cell transplantation patients
- PMID: 18398611
- PMCID: PMC6708594
- DOI: 10.1007/s00280-008-0748-0
A novel phenotypic method to determine fludarabine triphosphate accumulation in T-lymphocytes from hematopoietic cell transplantation patients
Abstract
Purpose: Fludarabine is an integral anticancer agent for patients with chronic lymphocytic leukemia (CLL) and those receiving conditioning regimens prior to allogeneic hematopoietic cell transplantation (HCT). An individual's response to fludarabine may be influenced by the amount of CD4(+) and CD8(+) T-lymphocyte suppression. Fludarabine undergoes cellular uptake and activation to form the cytotoxic metabolite, fludarabine triphosphate (F-ara-ATP).
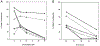
Methods: We have previously developed a highly sensitive LC-MS method to quantitate intracellular F-ara-ATP concentrations in a leukemic cell line. However, quantitation of F-ara-ATP concentrations within CD4(+) and CD8(+) T-lymphocytes from pharmacokinetic blood samples obtained from patients receiving fludarabine therapy is not feasible because of the limited number of T-lymphocytes that can be isolated from each blood sample. Thus, we sought to determine F-ara-ATP accumulation after ex vivo exposure of freshly isolated human CD4(+) or CD8(+) T-lymphocytes to fludarabine. The method was optimized in T-lymphocytes obtained from healthy volunteers, and proved to be a feasible method to determine F-ara-ATP accumulation in patients undergoing HCT.
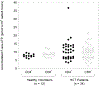
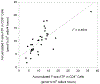
Results: Considerable variability was observed in F-ara-ATP accumulation in HCT patients (10.5- and 12.5-fold in CD4(+) and CD8(+) cells, respectively), compared to healthy volunteers (1.6- and 1.9-fold in CD4(+) and CD8(+) cells, respectively). Larger variability was also observed in gene expression of transporters and enzymes involved in F-ara-ATP accumulation in HCT patients; however, F-ara-ATP accumulation was not correlated with gene expression, which is in agreement with previous studies.
Conclusions: The quantitation of F-ara-ATP accumulation in T-lymphocytes provides a novel tool to evaluate patient sensitivity to fludarabine. This tool can be used in future studies to evaluate whether intracellular F-ara-ATP accumulation is associated with efficacy and/or toxicity in patients receiving fludarabine.
Figures

References
-
- Auletta JJ, Lazarus HM (2005) Immune restoration following hematopoietic stem cell transplantation: an evolving target. Bone Marrow Transplant 35: 835–57 - PubMed
-
- Baron F, Maris MB, Sandmaier BM, Storer BE, Sorror M, Diaconescu R, Woolfrey AE, Chauncey TR, Flowers ME, Mielcarek M, Maloney DG, Storb R (2005) Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol 23: 1993–2003 - PubMed
-
- Baron F, Maris MB, Storer BE, Sandmaier BM, Stuart MJ, McSweeney PA, Radich JP, Pulsipher MA, Agura ED, Chauncey TR, Maloney DG, Shizuru JA, Storb R (2005) HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with chronic myeloid leukemia. Biol Blood Marrow Transplant 11: 272–9 - PubMed
-
- Baron F, Sandmaier BM (2006) Chimerism and outcomes after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Leukemia 20: 1690–700 - PubMed
-
- Baron F, Storb R (2006) Allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning as treatment for hematologic malignancies and inherited blood disorders. Mol Ther 13: 26–41 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

