Silent Cerebral Infarct Transfusion (SIT) trial imaging core: application of novel imaging information technology for rapid and central review of MRI of the brain
- PMID: 18398653
- PMCID: PMC2801625
- DOI: 10.1007/s10278-008-9114-3
Silent Cerebral Infarct Transfusion (SIT) trial imaging core: application of novel imaging information technology for rapid and central review of MRI of the brain
Abstract
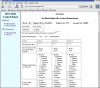
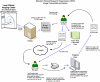
The Silent Cerebral Infarct Multicenter Transfusion (SIT) Trial is a multi-institutional intervention trial in which children with silent cerebral infarcts are randomized to receive either blood transfusion therapy or observation (standard care) for 36 months. The SIT Trial is scheduled to enroll approximately 1,880 children with sickle cell disease from 29 clinical sites in the United States, Canada, UK, and France. Each child undergoes a screening magnetic resonance imaging (MRI) of the brain to detect the presence of silent cerebral infarct-like lesions, a pre-randomization (baseline) MRI and exit MRI to determine if there are new or enlarged cerebral infarcts, using a designated, prospective imaging protocol. The objective of this manuscript is to describe the innovative method used to process and adjudicate imaging studies for an international trial with a primary endpoint that includes neuroimaging. Institution investigators at each site were provided with computer hardware and software for transmission of MRI images that allow them to strip the scans of all personal information and add unique study identifiers. Three neuroradiologists at separate academic centers review MRI studies and determine the presence or absence of silent cerebral infarct-like lesions. Their findings are subsequently placed on web-based case report forms and sent to the Statistical Coordinating Center. The average time from imaging center receipt of the MRI study to the radiology committee report back to the local site is less than two working days. This novel strategy was designed to maximize efficiency and minimize cost of a complex large multicenter trial that depends heavily on neuroimaging for entry criteria and assessment for the primary outcome measures. The technology, process, and expertise used in the SIT Trial can be adapted to virtually any clinical research trial with digital imaging requirements.
Figures










References
-
- Ashley-Koch A, Yang Q, Olney RS. Sickle hemoglobin (HbS) allele and sickle cell disease: a HuGE review. Am J Epidemiol. 2000;151:839–845. - PubMed
-
- Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288–294. - PubMed
-
- Buchanan GR, DeBaun MR, Quinn CT, Steinberg MH: Sickle Cell Disease. ASH Education Book. 35–47, 2004 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

