Phenotypic plasticity of neoplastic ovarian epithelium: unique cadherin profiles in tumor progression
- PMID: 18398687
- PMCID: PMC2836537
- DOI: 10.1007/s10585-008-9171-5
Phenotypic plasticity of neoplastic ovarian epithelium: unique cadherin profiles in tumor progression
Abstract
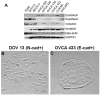
The mesodermally derived normal ovarian surface epithelium (OSE) displays both epithelial and mesenchymal characteristics and exhibits remarkable phenotypic plasticity during post-ovulatory repair. The majority of epithelial ovarian carcinomas (EOC) are derived from the OSE and represent the most lethal of all gynecological malignancies, as most patients (approximately 70%) present at diagnosis with disseminated intra-abdominal metastasis. The predominant pattern of EOC metastasis involves pelvic dissemination rather than lymphatic or hematologic spread, distinguishing EOC from other solid tumors. Acquisition of the metastatic phenotype involves a complex series of interrelated cellular events leading to dissociation (shedding) and dispersal of malignant cells. A key event in this process is disruption of cell-cell contacts via modulation of intercellular junctional components. In contrast to most carcinomas that downregulate E-cadherin expression during tumor progression, a unique feature of primary well-differentiated ovarian cancers is a gain of epithelial features, characterized by an increase in expression of E-cadherin. Subsequent reacquisition of mesenchymal features is observed in more advanced tumors with concomitant loss of E-cadherin expression and/or function during progression to metastasis. The functional consequences of this remarkable phenotypic plasticity are not fully understood, but may play a role in modulation of cell survival in suspension (ascites), chemoresistance, and intraperitoneal anchoring of metastatic lesions.
Figures




References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. Clin. 2007 Jan-Feb;57(1):43–66. - PubMed
-
- Wheeler JE. Pathology of malignant ovarian epithelial tumors and miscellaneous and rare ovarian and paraovarian neoplasms. In: Rubin SC, Sutton GP, editors. Ovarian Cancer. McGraw Hill; 1993. pp. 87–130.
-
- Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29. - PubMed
-
- DuBeau L. The cell or origin of ovarian epithelial tumors and the ovarian surface epithelium dogma: does the emperor have no clothes? Gyn Oncol. 1999;72:437–442. - PubMed
-
- Shedden KA, Kshisagar MP, Schwartz DR, Wu R, Yu H, Misek DE, Hanash S, Katabuchi H, Ellenson LH, Fearon ER, Cho KC. Histologic type, organ or origin and wnt pathway status: effect on gene expression in ovarian and uterine carcinomas. Clin Can Res. 2005;11:2123–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

