Evolution of decitabine development: accomplishments, ongoing investigations, and future strategies
- PMID: 18398832
- PMCID: PMC4784235
- DOI: 10.1002/cncr.23463
Evolution of decitabine development: accomplishments, ongoing investigations, and future strategies
Abstract
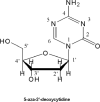
Decitabine (5-aza-2'-deoxycytidine) is a hypomethylating agent with a dual mechanism of action: reactivation of silenced genes and differentiation at low doses, and cytotoxicity at high doses. The original studies in the 1980s used decitabine as a classical anticancer drug, at its maximum clinically tolerated dose, 1500 to 2500 mg/m(2) per course. At these doses, decitabine was found to be active in leukemia, but was associated with delayed and prolonged myelosuppression. After a better understanding of epigenetics in cancer and the role of decitabine in epigenetic (hypomethylating) therapy was gained, it was reevaluated at approximately 1/20th of the previous doses (ie, at 'optimal biologic' doses that modulate hypomethylation). In these dose schedules of decitabine (100 to 150 mg/m(2) per course), the drug was found to be active with manageable side effects in patients with myelodysplastic syndromes (MDS) and other myeloid tumors. Optimizing dosing schedules of decitabine to maximize hypomethylation (low dose, high dose intensity, and multiple cycles) have further improved results, suggesting that decitabine is an active therapy that alters the natural course of MDS. Combination therapies that augment the epigenetic effect of decitabine will likely improve responses and extend its use for the treatment of other malignancies.
(c) 2008 American Cancer Society.
Similar articles
-
Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2'-deoxycytidine (decitabine) in hematopoietic malignancies.Blood. 2004 Mar 1;103(5):1635-40. doi: 10.1182/blood-2003-03-0687. Epub 2003 Nov 6. Blood. 2004. PMID: 14604977 Clinical Trial.
-
Pharmacokinetic evaluation of decitabine for the treatment of leukemia.Expert Opin Drug Metab Toxicol. 2011 May;7(5):661-72. doi: 10.1517/17425255.2011.575062. Expert Opin Drug Metab Toxicol. 2011. PMID: 21500965 Review.
-
Decitabine in the treatment of myelodysplastic syndromes.Expert Rev Anticancer Ther. 2010 Jan;10(1):9-22. doi: 10.1586/era.09.164. Expert Rev Anticancer Ther. 2010. PMID: 20014881 Review.
-
5-azacytidine and decitabine monotherapies of myelodysplastic disorders.Ann Pharmacother. 2005 Oct;39(10):1700-9. doi: 10.1345/aph.1E612. Epub 2005 Sep 6. Ann Pharmacother. 2005. PMID: 16144884 Review.
-
Clinical results with the DNA hypomethylating agent 5-aza-2'-deoxycytidine (decitabine) in patients with myelodysplastic syndromes: an update.Semin Hematol. 2012 Oct;49(4):330-41. doi: 10.1053/j.seminhematol.2012.08.001. Semin Hematol. 2012. PMID: 23079063 Review.
Cited by
-
Paradoxical whole genome DNA methylation dynamics of 5'aza-deoxycytidine in chronic low-dose exposure in mice.Epigenetics. 2021 Jan-Feb;16(2):209-227. doi: 10.1080/15592294.2020.1790951. Epub 2020 Jul 11. Epigenetics. 2021. PMID: 32619143 Free PMC article.
-
Efficacy of decitabine-loaded nanogels in overcoming cancer drug resistance is mediated via sustained DNA methyltransferase 1 (DNMT1) depletion.Cancer Lett. 2013 Apr 30;331(1):122-9. doi: 10.1016/j.canlet.2012.12.009. Epub 2013 Jan 7. Cancer Lett. 2013. PMID: 23305699 Free PMC article.
-
A semantic web framework to integrate cancer omics data with biological knowledge.BMC Bioinformatics. 2012 Jan 25;13 Suppl 1(Suppl 1):S10. doi: 10.1186/1471-2105-13-S1-S10. BMC Bioinformatics. 2012. PMID: 22373303 Free PMC article.
-
Prostaglandin E2 Reverses the Effects of DNA Methyltransferase Inhibitor and TGFB1 on the Conversion of Naive T Cells to iTregs.Transfus Med Hemother. 2020 Jun;47(3):244-253. doi: 10.1159/000502582. Epub 2019 Sep 19. Transfus Med Hemother. 2020. PMID: 32595429 Free PMC article.
-
Safety and clinical activity of 5-aza-2'-deoxycytidine (decitabine) with or without Hyper-CVAD in relapsed/refractory acute lymphocytic leukaemia.Br J Haematol. 2014 Nov;167(3):356-65. doi: 10.1111/bjh.13050. Epub 2014 Jul 26. Br J Haematol. 2014. PMID: 25066676 Free PMC article. Clinical Trial.
References
-
- List AF, Vardiman J, Issa JP, Dewitte TM. Myelodysplastic syndromes. Hematology. 2004:297–317. - PubMed
-
- Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068–1070. - PubMed
-
- Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. - PubMed
-
- Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation — a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–196. - PubMed
-
- Santini V, Kantarjian HM, Issa JP. Changes in DNA methylation in neoplasia: pathophysiology and therapeutic implications. Ann Intern Med. 2001;134:573–586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous