Molecular and functional characterization of a Rho GDP dissociation inhibitor in the filamentous fungus Tuber borchii
- PMID: 18400087
- PMCID: PMC2362126
- DOI: 10.1186/1471-2180-8-57
Molecular and functional characterization of a Rho GDP dissociation inhibitor in the filamentous fungus Tuber borchii
Abstract
Background: Small GTPases of the Rho family function as tightly regulated molecular switches that govern important cellular functions in eukaryotes. Several families of regulatory proteins control their activation cycle and subcellular localization. Members of the guanine nucleotide dissociation inhibitor (GDI) family sequester Rho GTPases from the plasma membrane and keep them in an inactive form.
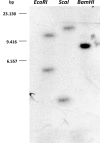
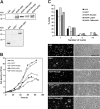
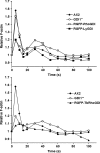
Results: We report on the characterization the RhoGDI homolog of Tuber borchii Vittad., an ascomycetous ectomycorrhizal fungus. The Tbgdi gene is present in two copies in the T. borchii genome. The predicted amino acid sequence shows high similarity to other known RhoGDIs. Real time PCR analyses revealed an increased expression of Tbgdi during the phase preparative to the symbiosis instauration, in particular after stimulation with root exudates extracts, that correlates with expression of Tbcdc42. In a translocation assay TbRhoGDI was able to solubilize TbCdc42 from membranes. Surprisingly, TbRhoGDI appeared not to interact with S. cerevisiae Cdc42, precluding the use of yeast as a surrogate model for functional studies. To study the role of TbRhoGDI we performed complementation experiments using a RhoGDI null strain of Dictyostelium discoideum, a model organism where the roles of Rho signaling pathways are well established. For comparison, complementation with mammalian RhoGDI1 and LyGDI was also studied in the null strain. Although interacting with Rac1 isoforms, TbRhoGDI was not able to revert the defects of the D. discoideum RhoGDI null strain, but displayed an additional negative effect on the cAMP-stimulated actin polymerization response.
Conclusion: T. borchii expresses a functional RhoGDI homolog that appears as an important modulator of cytoskeleton reorganization during polarized apical growth that antecedes symbiosis instauration. The specificity of TbRhoGDI actions was underscored by its inability to elicit a growth defect in S. cerevisiae or to compensate the loss of a D. discoideum RhoGDI. Knowledge of the cell signaling at the basis of cytoskeleton reorganization of ectomycorrhizal fungi is essential for improvements in the production of mycorrhized plant seedlings used in timberland extension programs and fruit body production.
Figures



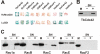

References
-
- Nehls U, Béguiristain T, Ditengou F, Lapeyrie F, Martin F. The expression of a symbiosis-regulated gene in eucalypt roots is regulated by auxins and hypaphorine, the tryptophan betaine of the ectomycorrhizal basidiomycete Pisolithus tinctorius. Planta. 1998;207:296–302. doi: 10.1007/s004250050486. - DOI - PubMed
-
- Podila GK, Zheng J, Balasubramanian S, Sundaram S, Hiremath S, Brand JH, Hymes MJ. Fungal gene expression in early symbiotic interaction between Laccaria bicolor and red pine. Plant Soil. 2002;244:117–128. doi: 10.1023/A:1020275330363. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

