Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo
- PMID: 18400162
- PMCID: PMC2390913
- DOI: 10.1016/j.neuron.2008.02.003
Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo
Abstract
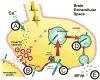
Aggregation of amyloid-beta (Abeta) peptide into soluble and insoluble forms within the brain extracellular space is central to the pathogenesis of Alzheimer's disease. Full-length amyloid precursor protein (APP) is endocytosed from the cell surface into endosomes where it is cleaved to produce Abeta. Abeta is subsequently released into the brain interstitial fluid (ISF). We hypothesized that synaptic transmission results in more APP endocytosis, thereby increasing Abeta generation and release into the ISF. We found that inhibition of clathrin-mediated endocytosis immediately lowers ISF Abeta levels in vivo. Two distinct methods that increased synaptic transmission resulted in an elevation of ISF Abeta levels. Inhibition of endocytosis, however, prevented the activity-dependent increase in Abeta. We estimate that approximately 70% of ISF Abeta arises from endocytosis-associated mechanisms, with the vast majority of this pool also dependent on synaptic activity. These findings have implications for AD pathogenesis and may provide insights into therapeutic intervention.
Figures


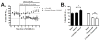



References
-
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. - PMC - PubMed
-
- Caccamo A, Oddo S, Billings LM, Green KN, Martinez-Coria H, Fisher A, LaFerla FM. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49:671–682. - PubMed
-
- Cam JA, Zerbinatti CV, Knisely JM, Hecimovic S, Li Y, Bu G. The low density lipoprotein receptor-related protein 1B retains beta-amyloid precursor protein at the cell surface and reduces amyloid-beta peptide production. J Biol Chem. 2004;279:29639–29646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS054174/NS/NINDS NIH HHS/United States
- R01 AG013956/AG/NIA NIH HHS/United States
- DA07261/DA/NIDA NIH HHS/United States
- AG13956/AG/NIA NIH HHS/United States
- R37 AG013956/AG/NIA NIH HHS/United States
- T32 DA007261/DA/NIDA NIH HHS/United States
- AG027924/AG/NIA NIH HHS/United States
- MH78823/MH/NIMH NIH HHS/United States
- P30 NS057105/NS/NINDS NIH HHS/United States
- K01 AG029524/AG/NIA NIH HHS/United States
- R01 AG027924/AG/NIA NIH HHS/United States
- R01 MH078823/MH/NIMH NIH HHS/United States
- NS54174/NS/NINDS NIH HHS/United States
- AG029524/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

