Regulation of motor neuron specification by phosphorylation of neurogenin 2
- PMID: 18400164
- PMCID: PMC2587148
- DOI: 10.1016/j.neuron.2008.01.037
Regulation of motor neuron specification by phosphorylation of neurogenin 2
Abstract
The mechanisms by which proneural basic helix-loop-helix (bHLH) factors control neurogenesis have been characterized, but it is not known how they specify neuronal cell-type identity. Here, we provide evidence that two conserved serine residues on the bHLH factor neurogenin 2 (Ngn2), S231 and S234, are phosphorylated during motor neuron differentiation. In knockin mice in which S231 and S234 of Ngn2 were mutated to alanines, neurogenesis occurs normally, but motor neuron specification is impaired. The phosphorylation of Ngn2 at S231 and S234 facilitates the interaction of Ngn2 with LIM homeodomain transcription factors to specify motor neuron identity. The phosphorylation-dependent cooperativity between Ngn2 and homeodomain transcription factors may be a general mechanism by which the activities of bHLH and homeodomain proteins are temporally and spatially integrated to generate the wide diversity of cell types that are a hallmark of the nervous system.
Figures
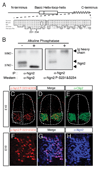
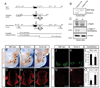



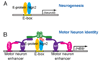
Comment in
-
Neurogenesis or neuronal specification: phosphorylation strikes again!Neuron. 2008 Apr 10;58(1):3-5. doi: 10.1016/j.neuron.2008.03.023. Neuron. 2008. PMID: 18400155 Review.
References
-
- Agulnick AD, Taira M, Breen JJ, Tanaka T, Dawid IB, Westphal H. Interactions of the LIM-domain-binding factor Ldb1 with LIM homeodomain proteins. Nature. 1996;384:270–272. - PubMed
-
- Andersson E, Jensen JB, Parmar M, Guillemot F, Bjorklund A. Development of the mesencephalic dopaminergic neuron system is compromised in the absence of neurogenin 2. Development. 2006;133:507–516. - PubMed
-
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. - PubMed
-
- Bach I, Carriere C, Ostendorff HP, Andersen B, Rosenfeld MG. A family of LIM domain-associated cofactors confer transcriptional synergism between LIM and Otx homeodomain proteins. Genes Dev. 1997;11:1370–1380. - PubMed
-
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

