A cascade of Ca(2+)/calmodulin-dependent protein kinases regulates the differentiation and functional activation of murine neutrophils
- PMID: 18400360
- PMCID: PMC2577899
- DOI: 10.1016/j.exphem.2008.02.009
A cascade of Ca(2+)/calmodulin-dependent protein kinases regulates the differentiation and functional activation of murine neutrophils
Abstract
Objective: The function of neutrophils as primary mediators of innate immunity depends on the activity of granule proteins and critical components of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex. Expression of their cognate genes is regulated during neutrophil differentiation by a complex network of intracellular signaling pathways. In this study, we have investigated the role of two members of the calcium/calmodulin-dependent protein kinase (CaMK) signaling cascade, CaMK I-like kinase (CKLiK) and CaMKKalpha, in regulating neutrophil differentiation and functional activation.
Materials and methods: Mouse myeloid cell lines were used to examine the expression of a CaMK cascade in developing neutrophils and to examine the effects of constitutive activation vs inhibition of CaMKs on neutrophil maturation.
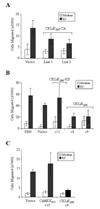
Results: Expression of CaMKKalpha was shown to increase during neutrophil differentiation in multiple cell lines, whereas expression of CKLiK increased as multipotent progenitors committed to promyelocytes, but then decreased as cells differentiated into mature neutrophils. Expression of constitutively active CKLiKs did not affect morphologic maturation, but caused dramatic decreases in both respiratory burst responses and chemotaxis. This loss of neutrophil function was accompanied by reduced secondary granule and gp91(phox) gene expression. The CaMK inhibitor KN-93 attenuated cytokine-stimulated proliferative responses in promyelocytic cell lines, and inhibited the respiratory burst. Similar data were observed with the CaMKKalpha inhibitor, STO-609.
Conclusions: Overactivation of a cascade of CaMKs inhibits neutrophil maturation, suggesting that these kinases play an antagonistic role during neutrophil differentiation, but at least one CaMK is required for myeloid cell expansion and functional activation.
Figures


Similar articles
-
Heterogeneity of functional responses in differentiated myeloid cell lines reveals EPRO cells as a valid model of murine neutrophil functional activation.J Leukoc Biol. 2005 May;77(5):669-79. doi: 10.1189/jlb.1004567. Epub 2005 Jan 26. J Leukoc Biol. 2005. PMID: 15673544
-
Activation of the aryl hydrocarbon receptor by the calcium/calmodulin-dependent protein kinase kinase inhibitor 7-oxo-7H-benzimidazo[2,1-a]benz[de]isoquinoline-3-carboxylic acid (STO-609).Drug Metab Dispos. 2008 Dec;36(12):2556-63. doi: 10.1124/dmd.108.023333. Epub 2008 Aug 28. Drug Metab Dispos. 2008. PMID: 18755850
-
Pleiotropic roles of Ca+2/calmodulin-dependent pathways in regulating cadmium-induced toxicity in human osteoblast-like cell lines.Toxicol Lett. 2016 Oct 17;260:18-27. doi: 10.1016/j.toxlet.2016.08.020. Epub 2016 Aug 21. Toxicol Lett. 2016. PMID: 27558804 Free PMC article.
-
Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane.Semin Immunopathol. 2008 Jul;30(3):279-89. doi: 10.1007/s00281-008-0118-3. Epub 2008 Jun 7. Semin Immunopathol. 2008. PMID: 18536919 Review.
-
The roles of NADPH oxidase in modulating neutrophil effector responses.Mol Oral Microbiol. 2019 Apr;34(2):27-38. doi: 10.1111/omi.12252. Epub 2019 Feb 7. Mol Oral Microbiol. 2019. PMID: 30632295 Free PMC article. Review.
Cited by
-
Inhibition of Calcium/Calmodulin-Dependent Protein Kinase Kinase β Is Detrimental in Hypoxia⁻Ischemia Neonatal Brain Injury.Int J Mol Sci. 2019 Apr 26;20(9):2063. doi: 10.3390/ijms20092063. Int J Mol Sci. 2019. PMID: 31027360 Free PMC article.
-
LncRNA ILF3-AS1 Promotes the Progression of Colon Adenocarcinoma Cells Through the miR-619-5p/CAMK1D Axis.Onco Targets Ther. 2021 Mar 12;14:1861-1872. doi: 10.2147/OTT.S296441. eCollection 2021. Onco Targets Ther. 2021. PMID: 33737811 Free PMC article.
-
Cooperative Activity of GABP with PU.1 or C/EBPε Regulates Lamin B Receptor Gene Expression, Implicating Their Roles in Granulocyte Nuclear Maturation.J Immunol. 2016 Aug 1;197(3):910-22. doi: 10.4049/jimmunol.1402285. Epub 2016 Jun 24. J Immunol. 2016. PMID: 27342846 Free PMC article.
-
Neutrophils promote T-cell activation through the regulated release of CD44-bound Galectin-9 from the cell surface during HIV infection.PLoS Biol. 2021 Aug 19;19(8):e3001387. doi: 10.1371/journal.pbio.3001387. eCollection 2021 Aug. PLoS Biol. 2021. PMID: 34411088 Free PMC article.
-
A cell-intrinsic role for CaMKK2 in granulocyte lineage commitment and differentiation.J Leukoc Biol. 2011 Nov;90(5):897-909. doi: 10.1189/jlb.0311152. Epub 2011 Aug 4. J Leukoc Biol. 2011. PMID: 21816924 Free PMC article.
References
-
- Liu H, Pope RM. Phagocytes: Mechanisms of inflammation and tissue destruction. Rheum. Dis. Clin. North Am. 2004;30:19–39. - PubMed
-
- Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc. Res. 2002;53:31–47. - PubMed
-
- Soderling TR. The ca-calmodulin-dependent protein kinase cascade. Trends Biochem. Sci. 1999;24:232–236. - PubMed
-
- Corcoran EE, Means AR. Defining Ca2+/calmodulin-dependent protein kinase cascades in transcriptional regulation. J. Biol. Chem. 2001;276:2975–2978. - PubMed
-
- Goldberg J, Nairn AC, Kuriyan J. Structural basis for the autoinhibition of calcium/calmodulin-dependent protein kinase I. Cell. 1996;84:875–887. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

