Priming with EGFR tyrosine kinase inhibitor and EGF sensitizes ovarian cancer cells to respond to chemotherapeutical drugs
- PMID: 18400375
- PMCID: PMC2519958
- DOI: 10.1016/j.canlet.2008.02.062
Priming with EGFR tyrosine kinase inhibitor and EGF sensitizes ovarian cancer cells to respond to chemotherapeutical drugs
Abstract
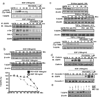
Over-expression of EGFR, as in most cases of ovarian cancer, is associated with advanced-stage disease and poor prognosis. Activation of EGFR signaling pathway is involved in increased cell proliferation, angiogenesis, metastasis and decreased apoptosis. Tyrosine kinase activity is essential for signal transduction and receptor down-regulation. However, we found in this study that tyrosine kinase activity is not necessary in ligand-induced EGFR down-regulation in ovarian cancer cell line CaOV3 cells. EGFR tyrosine kinase inhibitors, such as PD153035, AG1478, as well as non-specific tyrosine kinase inhibitor PP2 cannot reverse EGF-induced down-regulation of EGFR. These findings thus permit us to develop the following exciting but unconventional strategy to sensitize cancer cells, namely, by priming ovarian cancer cells with EGF and EGFR inhibitor PD153035, before chemotherapy. This priming procedure down-regulates EGFR without induction of mitogenic signals such as ERK and PI3K/AKT. EGF plus EGFR inhibitor-primed ovarian cancer cells display increased sensitivity to taxol-induced cell death, resistant to EGF-induced cell migration and cell proliferation as well as ERK and PI3K/AKT activation. Further studies showed that PD153035, which does not reverse ligand-induced EGFR down-regulation, blocks EGF-induced EGFR activation as well as EGFR's binding to c-cbl and Grb2. Taken together, we contend that priming with EGFR inhibitors plus EGF inhibits cell signaling pathways leading to cell proliferation and survival, while down-regulating EGFR. This priming approach sensitizes ovarian cancer cells and would ultimately result in better chemotherapeutical outcome.
Figures





References
-
- Chan JK, Pham H, You XJ, Cloven NG, Burger RA, Rose GS, Van Nostrand K, Korc M, Disaia PJ, Fan H. Suppression of ovarian cancer cell tumorigenicity and evasion of Cisplatin resistance using a truncated epidermal growth factor receptor in a rat model. Cancer Res. 2005;65:3243–3248. - PubMed
-
- Berchuck A, Rodriguez GC, Kamel A, Dodge RK, Soper JT, Clarke-Pearson DL, Bast RC., Jr Epidermal growth factor receptor expression in normal ovarian epithelium and ovarian cancer. I. Correlation of receptor expression with prognostic factors in patients with ovarian cancer. Am. J. Obstet. Gynecol. 1991;164:669–674. - PubMed
-
- Maihle NJ, Baron AT, Barrette BA, Boardman CH, Christensen TA, Cora EM, Faupel-Badger JM, Greenwood T, Juneja SC, Lafky JM, Lee H, Reiter JL, Podratz KC. EGF/ErbB receptor family in ovarian cancer. Cancer Treat. Res. 2002;107:247–258. - PubMed
-
- Morishige K, Kurachi H, Amemiya K, Fujita Y, Yamamoto T, Miyake A, Tanizawa O. Evidence for the involvement of transforming growth factor alpha and epidermal growth factor receptor autocrine growth mechanism in primary human ovarian cancers in vitro. Cancer Res. 1991;51:5322–5328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

