The Hsp60-(p.V98I) mutation associated with hereditary spastic paraplegia SPG13 compromises chaperonin function both in vitro and in vivo
- PMID: 18400758
- PMCID: PMC3259655
- DOI: 10.1074/jbc.M800548200
The Hsp60-(p.V98I) mutation associated with hereditary spastic paraplegia SPG13 compromises chaperonin function both in vitro and in vivo
Abstract
We have previously reported the association of a mutation (c.292G > A/p.V98I) in the human HSPD1 gene that encodes the mitochondrial Hsp60 chaperonin with a dominantly inherited form of hereditary spastic paraplegia. Here, we show that the purified Hsp60-(p.V98I) chaperonin displays decreased ATPase activity and exhibits a strongly reduced capacity to promote folding of denatured malate dehydrogenase in vitro. To test its in vivo functions, we engineered a bacterial model system that lacks the endogenous chaperonin genes and harbors two plasmids carrying differentially inducible operons with human Hsp10 and wild-type Hsp60 or Hsp10 and Hsp60-(p.V98I), respectively. Ten hours after shutdown of the wild-type chaperonin operon and induction of the Hsp60-(p.V98I)/Hsp10 mutant operon, bacterial cell growth was strongly inhibited. No globally increased protein aggregation was observed, and microarray analyses showed that a number of genes involved in metabolic pathways, some of which are essential for robust aerobic growth, were strongly up-regulated in Hsp60-(p.V98I)-expressing bacteria, suggesting that the growth arrest was caused by defective folding of some essential proteins. Co-expression of Hsp60-(p.V98I) and wild-type Hsp60 exerted a dominant negative effect only when the chaperonin genes were expressed at relatively low levels. Based on our in vivo and in vitro data, we propose that the major effect of heterozygosity for the Hsp60-(p.V98I) mutation is a moderately decreased activity of chaperonin complexes composed of mixed wild-type and Hsp60-(p.V98I) mutant subunits.
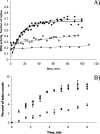
Figures





References
-
- Fink, J. K. (2006) Curr. Neurol. Neurosci. Rep. 6 65–76 - PubMed
-
- Deluca, G. C., Ebers, G. C., and Esiri, M. M. (2004) Neuropathol. Appl. Neurobiol. 30 576–584 - PubMed
-
- Depienne, C., Stevanin, G., Brice, A., and Durr, A. (2007) Curr. Opin. Neurol. 20 674–680 - PubMed
-
- Bross, P., Rugarli, E. I., Casari, G., and Langer, T. (2004) in Mitochondrial Function and Genetics (Koehler, C., and Bauer, M., eds) pp. 97–121, Springer, Heidelberg
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

