A subgenomic segment of Theiler's murine encephalomyelitis virus RNA causes demyelination
- PMID: 18400855
- PMCID: PMC2395141
- DOI: 10.1128/JVI.02432-07
A subgenomic segment of Theiler's murine encephalomyelitis virus RNA causes demyelination
Abstract
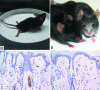
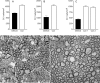
The DA strain of Theiler's murine encephalomyelitis virus (TMEV) causes a persistent central nervous system (CNS) infection of mice with a restricted virus gene expression and induces an inflammatory demyelinating disease that is thought to be immune mediated and a model of multiple sclerosis (MS). The relative contribution of virus vis-à-vis the immune system in the pathogenesis of DA-induced white matter disease remains unclear, as is also true in MS. To clarify the pathogenesis of DA-induced demyelination, we used Cre/loxP technology to generate a transgenic mouse that has tamoxifen (Tm)-inducible expression of a subgenomic segment of DA RNA in oligodendrocytes and Schwann cells. Tm-treated young transgenic mice developed progressive weakness leading to death, with abnormalities of oligodendrocytes and Schwann cells and demyelination, but without inflammation, demonstrating that DA virus can play a direct pathogenic role in demyelination. Tm treatment of mice at a later age resulted in milder disease, with evidence of peripheral nerve remyelination and focal fur depigmentation; surviving weak mice had persistent expression of the recombined transgene in the CNS, suggesting that the DA subgenomic segment can cause cellular dysfunction but not death, possibly similar to the situation seen during DA virus persistence. These studies demonstrate that DA RNA or a DA protein(s) is toxic to myelin-synthesizing cells. This Cre/loxP transgenic system allows for spatially and temporally controlled expression of the viral transgene and is valuable for clarifying nonimmune (and immune) mechanisms of demyelination induced by TMEV as well as other viruses.
Figures




References
-
- Chen, H. H., W. P. Kong, L. Zhang, P. L. Ward, and R. P. Roos. 1995. A picornaviral protein synthesized out of frame with the polyprotein plays a key role in a virus-induced immune-mediated demyelinating disease. Nat. Med. 1927-931. - PubMed
-
- Doerflinger, N. H., W. B. Macklin, and B. Popko. 2003. Inducible site-specific recombination in myelinating cells. Genesis 3563-72. - PubMed
-
- Dye, M. J., and N. J. Proudfoot. 2001. Multiple transcript cleavage precedes polymerase release in termination by RNA polymerase II. Cell 105669-681. - PubMed
-
- Dye, M. J., and N. J. Proudfoot. 1999. Terminal exon definition occurs cotranscriptionally and promotes termination of RNA polymerase II. Mol. Cell 3371-378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

