Spectrotemporal processing differences between auditory cortical fast-spiking and regular-spiking neurons
- PMID: 18400888
- PMCID: PMC2474630
- DOI: 10.1523/JNEUROSCI.5366-07.2008
Spectrotemporal processing differences between auditory cortical fast-spiking and regular-spiking neurons
Abstract
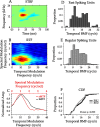
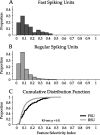
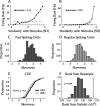
Excitatory pyramidal neurons and inhibitory interneurons constitute the main elements of cortical circuitry and have distinctive morphologic and electrophysiological properties. Here, we differentiate them by analyzing the time course of their action potentials (APs) and characterizing their receptive field properties in auditory cortex. Pyramidal neurons have longer APs and discharge as regular-spiking units (RSUs), whereas basket and chandelier cells, which are inhibitory interneurons, have shorter APs and are fast-spiking units (FSUs). To compare these neuronal classes, we stimulated cat primary auditory cortex neurons with a dynamic moving ripple stimulus and constructed single-unit spectrotemporal receptive fields (STRFs) and their associated nonlinearities. FSUs had shorter latencies, broader spectral tuning, greater stimulus specificity, and higher temporal precision than RSUs. The STRF structure of FSUs was more separable, suggesting more independence between spectral and temporal processing regimens. The nonlinearities associated with the two cell classes were indicative of higher feature selectivity for FSUs. These global functional differences between RSUs and FSUs suggest fundamental distinctions between putative excitatory and inhibitory interneurons that shape auditory cortical processing.
Figures










References
-
- Aertsen A, Johannesma PI. Spectro-temporal receptive fields of auditory neurons in the grassfrog. I. Characterization of tonal and natural stimuli. Biol Cybern. 1980;38:223–234. - PubMed
-
- Aguera y Arcas B, Fairhall AL, Bialek W. Computation in a single neuron: Hodgkin and Huxley revisited. Neural Comput. 2003;15:1715–1749. - PubMed
-
- Andermann ML, Ritt J, Neimark MA, Moore CI. Neural correlates of vibrissa resonance; band-pass and somatotopic representation of high-frequency stimuli. Neuron. 2004;42:451–463. - PubMed
-
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. - PubMed
-
- Azouz R, Gray CM, Nowak LG, McCormick DA. Physiological properties of inhibitory interneurons in cat striate cortex. Cereb Cortex. 1997;7:534–545. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
