The distinct temporal origins of olfactory bulb interneuron subtypes
- PMID: 18400896
- PMCID: PMC2505353
- DOI: 10.1523/JNEUROSCI.5625-07.2008
The distinct temporal origins of olfactory bulb interneuron subtypes
Abstract
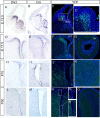
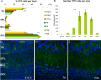
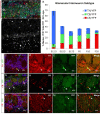
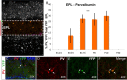
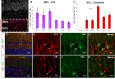
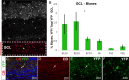
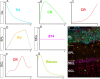
Olfactory bulb (OB) interneurons are a heterogeneous population produced beginning in embryogenesis and continuing through adulthood. Understanding how this diversity arises will provide insight into how OB microcircuitry is established as well as adult neurogenesis. Particular spatial domains have been shown to contribute specific interneuron subtypes. However, the temporal profile by which OB interneuron subtypes are produced is unknown. Using inducible genetic fate mapping of Dlx1/2 precursors, we analyzed the production of seven OB interneuron subtypes and found that the generation of each subpopulation has a unique temporal signature. Within the glomerular layer, the production of tyrosine hydroxylase-positive interneurons is maximal during early embryogenesis and decreases thereafter. In contrast, the generation of calbindin interneurons is maximal during late embryogenesis and declines postnatally, whereas calretinin (CR) cell production is low during embryogenesis and increases postnatally. Parvalbumin interneurons within the external plexiform layer are produced only perinatally, whereas the generation of 5T4-positive granule cells in the mitral cell layer does not change significantly over time. CR-positive granule cells are not produced at early embryonic time points, but constitute a large percentage of the granule cells born after birth. Blanes cells in contrast are produced in greatest number during embryogenesis. Together we provide the first comprehensive analysis of the temporal generation of OB interneuron subtypes and demonstrate that the timing by which these populations are produced is tightly orchestrated.
Figures


Comment in
-
Temporal patterns of olfactory bulb interneuron neurogenesis.J Neurosci. 2008 Aug 13;28(33):8145-7. doi: 10.1523/JNEUROSCI.2321-08.2008. J Neurosci. 2008. PMID: 18701675 Free PMC article. Review. No abstract available.
References
-
- Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JL. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997;19:27–37. - PubMed
-
- Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT. Centre-surround inhibition among olfactory bulb glomeruli. Nature. 2003;426:623–629. - PubMed
-
- Bayer SA. 3H-thymidine-radiographic studies of neurogenesis in the rat olfactory bulb. Exp Brain Res. 1983;50:329–340. - PubMed
-
- Betarbet R, Zigova T, Bakay RA, Luskin MB. Dopaminergic and GABAergic interneurons of the olfactory bulb are derived from the neonatal subventricular zone. Int J Dev Neurosci. 1996;14:921–930. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
