Trafficking of membrane-associated proteins to cone photoreceptor outer segments requires the chromophore 11-cis-retinal
- PMID: 18400900
- PMCID: PMC2562706
- DOI: 10.1523/JNEUROSCI.0317-08.2008
Trafficking of membrane-associated proteins to cone photoreceptor outer segments requires the chromophore 11-cis-retinal
Erratum in
- J Neurosci. 2008 Nov 5;28(45):11741
Abstract
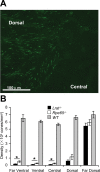
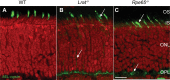
Lecithin retinol acyl transferase (LRAT) and retinal pigment epithelium protein 65 (RPE65) are key enzymes of the retinoid cycle. In Lrat(-/-) and Rpe65(-/-) mice, models of human Leber congenital amaurosis, the retinoid cycle is disrupted and 11-cis-retinal, the chromophore of visual pigments, is not produced. The Lrat(-/-) and Rpe65(-/-) retina phenotype presents with rapid sectorial cone degeneration, and the visual pigments, S-opsin and M/L-opsin, fail to traffic to cone outer segments appropriately. In contrast, rod opsin traffics normally in mutant rods. Concomitantly, guanylate cyclase 1, cone T alpha-subunit, cone phosphodiesterase 6alpha' (PDE6alpha'), and GRK1 (G-protein-coupled receptor kinase 1; opsin kinase) are not transported to Lrat(-/-) and Rpe65(-/-) cone outer segments. Aberrant localization of these membrane-associated proteins was evident at postnatal day 15, before the onset of ventral and central cone degeneration. Protein levels of cone T alpha and cone PDE6alpha' were reduced, whereas their transcript levels were unchanged, suggesting posttranslational degradation. In an Rpe65(-/-)Rho(-/-) double knock-out model, trafficking of cone pigments and membrane-associated cone phototransduction polypeptides to the outer segments proceeded normally after 11-cis-retinal administration. These results suggest that ventral and central cone opsins must be regenerated with 11-cis-retinal to permit transport to the outer segments. Furthermore, the presence of 11-cis-retinal is essential for proper transport of several membrane-associated cone phototransduction polypeptides in these cones.
Figures



Similar articles
-
Rpe65-/- and Lrat-/- mice: comparable models of leber congenital amaurosis.Invest Ophthalmol Vis Sci. 2008 Jun;49(6):2384-9. doi: 10.1167/iovs.08-1727. Epub 2008 Feb 22. Invest Ophthalmol Vis Sci. 2008. PMID: 18296659 Free PMC article.
-
Loss of cone photoreceptors caused by chromophore depletion is partially prevented by the artificial chromophore pro-drug, 9-cis-retinyl acetate.Hum Mol Genet. 2009 Jun 15;18(12):2277-87. doi: 10.1093/hmg/ddp163. Epub 2009 Apr 1. Hum Mol Genet. 2009. PMID: 19339306 Free PMC article.
-
Ciliary neurotrophic factor (CNTF) protects retinal cone and rod photoreceptors by suppressing excessive formation of the visual pigments.J Biol Chem. 2018 Sep 28;293(39):15256-15268. doi: 10.1074/jbc.RA118.004008. Epub 2018 Aug 16. J Biol Chem. 2018. PMID: 30115683 Free PMC article.
-
A model for transport of membrane-associated phototransduction polypeptides in rod and cone photoreceptor inner segments.Vision Res. 2008 Feb;48(3):442-52. doi: 10.1016/j.visres.2007.08.020. Epub 2007 Oct 18. Vision Res. 2008. PMID: 17949773 Free PMC article. Review.
-
Vitamin A and Vision.Subcell Biochem. 2016;81:231-259. doi: 10.1007/978-94-024-0945-1_9. Subcell Biochem. 2016. PMID: 27830507 Review.
Cited by
-
M-opsin protein degradation is inhibited by MG-132 in Rpe65⁻/⁻ retinal explant culture.Mol Vis. 2012;18:1516-25. Epub 2012 Jun 13. Mol Vis. 2012. PMID: 22736942 Free PMC article.
-
Expression of red/green-cone opsin mutants K82E, P187S, M273K result in unique pathobiological perturbations to cone structure and function.Front Neurosci. 2024 Feb 12;18:1368089. doi: 10.3389/fnins.2024.1368089. eCollection 2024. Front Neurosci. 2024. PMID: 38410159 Free PMC article.
-
Deletion of M-Opsin Prevents M Cone Degeneration in a Mouse Model of Leber Congenital Amaurosis.Am J Pathol. 2020 May;190(5):1059-1067. doi: 10.1016/j.ajpath.2020.01.005. Epub 2020 Feb 18. Am J Pathol. 2020. PMID: 32084365 Free PMC article.
-
Pathophysilogical mechanism and treatment strategies for Leber congenital amaurosis.Adv Exp Med Biol. 2014;801:791-6. doi: 10.1007/978-1-4614-3209-8_99. Adv Exp Med Biol. 2014. PMID: 24664772 Free PMC article.
-
Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics.Proc Natl Acad Sci U S A. 2008 Sep 30;105(39):15112-7. doi: 10.1073/pnas.0807027105. Epub 2008 Sep 22. Proc Natl Acad Sci U S A. 2008. PMID: 18809924 Free PMC article. Clinical Trial.
References
-
- Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J Biol Chem. 2004;279:49447–49454. - PubMed
-
- Coffey PJ, Gias C, McDermott CJ, Lundh P, Pickering MC, Sethi C, Bird A, Fitzke FW, Maass A, Chen LL, Holder GE, Luthert PJ, Salt TE, Moss SE, Greenwood J. Complement factor H deficiency in aged mice causes retinal abnormalities and visual dysfunction. Proc Natl Acad Sci USA. 2007;104:16651–16656. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY009339/EY/NEI NIH HHS/United States
- EY13520/EY/NEI NIH HHS/United States
- EY08123/EY/NEI NIH HHS/United States
- R24 EY014793/EY/NEI NIH HHS/United States
- EY14793/EY/NEI NIH HHS/United States
- C06 RR015455/RR/NCRR NIH HHS/United States
- CO6 RR015455/CO/NCI NIH HHS/United States
- EY09339/EY/NEI NIH HHS/United States
- R01 EY013520/EY/NEI NIH HHS/United States
- R01 EY004939/EY/NEI NIH HHS/United States
- EY014800-039003/EY/NEI NIH HHS/United States
- EY04939/EY/NEI NIH HHS/United States
- R01 EY008123/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
