R-Spondin family members regulate the Wnt pathway by a common mechanism
- PMID: 18400942
- PMCID: PMC2397303
- DOI: 10.1091/mbc.e08-02-0187
R-Spondin family members regulate the Wnt pathway by a common mechanism
Abstract
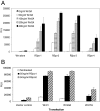
The R-Spondin (RSpo) family of secreted proteins is implicated in the activation of the Wnt signaling pathway. Despite the high structural homology between the four members, expression patterns and phenotypes in knockout mice have demonstrated striking differences. Here we dissected and compared the molecular and cellular function of all RSpo family members. Although all four RSpo proteins activate the canonical Wnt pathway, RSpo2 and 3 are more potent than RSpo1, whereas RSpo4 is relatively inactive. All RSpo members require Wnt ligands and LRP6 for activity and amplify signaling of Wnt3A, Wnt1, and Wnt7A, suggesting that RSpo proteins are general regulators of canonical Wnt signaling. Like RSpo1, RSpo2-4 antagonize DKK1 activity by interfering with DKK1 mediated LRP6 and Kremen association. Analysis of RSpo deletion mutants indicates that the cysteine-rich furin domains are sufficient and essential for the amplification of Wnt signaling and inhibition of DKK1, suggesting that Wnt amplification by RSpo proteins may be a direct consequence of DKK1 inhibition. Together, these findings indicate that RSpo proteins modulate the Wnt pathway by a common mechanism and suggest that coexpression with specific Wnt ligands and DKK1 may determine their biological specificity in vivo.
Figures


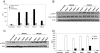



References
-
- Aoki M., Mieda M., Ikeda T., Hamada Y., Nakamura H., Okamoto H. R-spondin3 is required for mouse placental development. Dev. Biol. 2006;301:218–226. - PubMed
-
- Banziger C., Soldini D., Schutt C., Zipperlen P., Hausmann G., Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. - PubMed
-
- Behrens J., Jerchow B. A., Wurtele M., Grimm J., Asbrand C., Wirtz R., Kuhl M., Wedlich D., Birchmeier W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280(5363):596–599. - PubMed
-
- Bejsovec A. Wnt pathway activation: new relations and locations. Cell. 2005;120:11–14. - PubMed
-
- Bell S. M., Schreiner C. M., Hess K. A., Anderson K. P., Scott W. J. Asymmetric limb malformations in a new transgene insertional mutant, footless. Mech. Dev. 2003;120:597–605. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

