Purification and reconstitution of sterol transfer by native mouse ABCG5 and ABCG8
- PMID: 18402465
- PMCID: PMC2707165
- DOI: 10.1021/bi800292v
Purification and reconstitution of sterol transfer by native mouse ABCG5 and ABCG8
Abstract
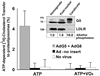
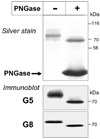
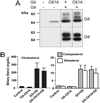
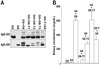
ABCG5 (G5) and ABCG8 (G8) are ATP-binding cassette half-transporters that limit intestinal uptake and promote biliary secretion of neutral sterols. Here, we describe the purification of endogenous G5G8 from mouse liver to near homogeneity. We incorporated the native proteins into membrane vesicles and reconstituted sterol transfer. Native gel electrophoresis, density-gradient ultracentrifugation, and chemical cross-linking studies indicated that the functional native complex is a heterodimer. No higher order oligomeric forms were observed at any stage in the catalytic cycle. Sterol transfer activity by purified native G5G8 was stable, stereospecific, and selective. We also report that G5 but not G8 is S-palmitoylated and that palmitoylation is not essential for dimerization, trafficking, or biliary sterol secretion. Both G5 and G8 have short but highly conserved cytoplasmic tails. The functional roles of these C-terminal regions were examined using an in vivo functional assay.
Figures






Similar articles
-
ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion.J Biol Chem. 2003 Nov 28;278(48):48275-82. doi: 10.1074/jbc.M310223200. Epub 2003 Sep 22. J Biol Chem. 2003. PMID: 14504269
-
Functional asymmetry of nucleotide-binding domains in ABCG5 and ABCG8.J Biol Chem. 2006 Feb 17;281(7):4507-16. doi: 10.1074/jbc.M512277200. Epub 2005 Dec 12. J Biol Chem. 2006. PMID: 16352607
-
Sequences in the nonconsensus nucleotide-binding domain of ABCG5/ABCG8 required for sterol transport.J Biol Chem. 2011 Mar 4;286(9):7308-14. doi: 10.1074/jbc.M110.210880. Epub 2011 Jan 5. J Biol Chem. 2011. PMID: 21209088 Free PMC article.
-
Association of ABCG5 and ABCG8 Transporters with Sitosterolemia.Adv Exp Med Biol. 2024;1440:31-42. doi: 10.1007/978-3-031-43883-7_2. Adv Exp Med Biol. 2024. PMID: 38036873 Review.
-
ABCG5/G8: a structural view to pathophysiology of the hepatobiliary cholesterol secretion.Biochem Soc Trans. 2019 Oct 31;47(5):1259-1268. doi: 10.1042/BST20190130. Biochem Soc Trans. 2019. PMID: 31654053 Free PMC article. Review.
Cited by
-
Oligomerization of human ATP-binding cassette transporters and its potential significance in human disease.Expert Opin Drug Metab Toxicol. 2009 Sep;5(9):1049-63. doi: 10.1517/17425250903124371. Expert Opin Drug Metab Toxicol. 2009. PMID: 19637987 Free PMC article. Review.
-
The role of Arabidopsis ABCG9 and ABCG31 ATP binding cassette transporters in pollen fitness and the deposition of steryl glycosides on the pollen coat.Plant Cell. 2014 Jan;26(1):310-24. doi: 10.1105/tpc.113.118935. Epub 2014 Jan 28. Plant Cell. 2014. PMID: 24474628 Free PMC article.
-
ABCG transporters and disease.FEBS J. 2011 Sep;278(18):3215-25. doi: 10.1111/j.1742-4658.2011.08171.x. Epub 2011 Jun 13. FEBS J. 2011. PMID: 21554546 Free PMC article. Review.
-
Cholesterol efflux mechanism revealed by structural analysis of human ABCA1 conformational states.Nat Cardiovasc Res. 2022;1(3):238-245. doi: 10.1038/s44161-022-00022-y. Epub 2022 Mar 3. Nat Cardiovasc Res. 2022. PMID: 37181814 Free PMC article.
-
Enzymatic trans-bilayer lipid transport: Mechanisms, efficiencies, slippage, and membrane curvature.Biochim Biophys Acta Biomembr. 2021 Mar 1;1863(3):183534. doi: 10.1016/j.bbamem.2020.183534. Epub 2020 Dec 17. Biochim Biophys Acta Biomembr. 2021. PMID: 33340491 Free PMC article. Review.
References
-
- Graf GA, Yu L, Li WP, Gerard R, Tuma PL, Cohen JC, Hobbs HH. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J. Biol. Chem. 2003;278:48275–48282. - PubMed
-
- Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

