Regulation of smooth muscle excitation and contraction
- PMID: 18402641
- PMCID: PMC8320329
- DOI: 10.1111/j.1365-2982.2008.01108.x
Regulation of smooth muscle excitation and contraction
Abstract
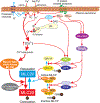
Smooth muscle cells (SMC) make up the muscular portion of the gastrointestinal (GI) tract from the distal oesophagus to the internal anal sphincter. Coordinated contractions of these cells produce the motor patterns of GI motility. Considerable progress was made during the last 20 years to understand the basic mechanisms controlling excitation-contraction (E-C) coupling. The smooth muscle motor is now understood in great molecular detail, and much has been learned about the mechanisms that deliver and recover Ca2+ during contractions. The majority of Ca2+ that initiates contractions comes from the external solution and is supplied by voltage-dependent Ca2+ channels (VDCC). VDCC are regulated largely by the effects of K+ and non-selective cation conductances (NSCC) on cell membrane potential and excitability. Ca2+ entry is supplemented by release of Ca2+ from IP(3) receptor-operated stores and by mechanisms that alter the sensitivity of the contractile apparatus to changes in cytoplasmic Ca2+. Molecular studies of the regulation of smooth muscle have been complicated by the plasticity of SMC and difficulties in culturing these cells without dramatic phenotypic changes. Major questions remain to be resolved regarding the details of E-C coupling in human GI smooth muscles. New discoveries regarding molecular expression that give GI smooth muscle their unique properties, the phenotypic changes that occur in SMC in GI motor disorders, tissue engineering approaches to repair or replace defective muscular regions, and molecular manipulations of GI smooth muscles in animals models and in cell culture will be topics for exciting investigations in the future.
Conflict of interest statement
CONFLICTS OF INTEREST
KMS was supported in this review by grants from the NIDDK: R37 DK40569 and P01 DK41315.
Figures

References
-
- Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol 2006; 68: 307–43. - PubMed
-
- Farrugia G. Role of interstitial cells of Cajal in health and disease. Neurogastroenterol Motil 2008; 20 (Suppl. 1): 54–63. - PubMed
-
- Sobue K, Hayashi K, Nishida W. Expressional regulation of smooth muscle cell-specific genes in association with phenotypic modulation. Mol Cell Biochem 1999; 190: 105–18. - PubMed
-
- Angstenberger M, Wegener JW, Pichler BJ et al. Severe intestinal obstruction on induced smooth muscle-specific ablation of the transcription factor SRF in adult mice. Gastroenterology 2007; 133: 1948–59. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

