Epiphytic bacterial community composition on two common submerged macrophytes in brackish water and freshwater
- PMID: 18402668
- PMCID: PMC2386815
- DOI: 10.1186/1471-2180-8-58
Epiphytic bacterial community composition on two common submerged macrophytes in brackish water and freshwater
Abstract
Background: Plants and their heterotrophic bacterial biofilm communities possibly strongly interact, especially in aquatic systems. We aimed to ascertain whether different macrophytes or their habitats determine bacterial community composition. We compared the composition of epiphytic bacteria on two common aquatic macrophytes, the macroalga Chara aspera Willd. and the angiosperm Myriophyllum spicatum L., in two habitats, freshwater (Lake Constance) and brackish water (Schaproder Bodden), using fluorescence in situ hybridization. The bacterial community composition was analysed based on habitat, plant species, and plant part.
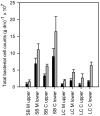
Results: The bacterial abundance was higher on plants from brackish water [5.3 x 10(7) cells (g dry mass)-1] than on plants from freshwater [1.3 x 10(7) cells (g dry mass)-1], with older shoots having a higher abundance. The organic content of freshwater plants was lower than that of brackish water plants (35 vs. 58%), and lower in C. aspera than in M. spicatum (41 vs. 52%). The content of nutrients, chlorophyll, total phenolic compounds, and anthocyanin differed in the plants and habitats. Especially the content of total phenolic compounds and anthocyanin was higher in M. spicatum, and in general higher in the freshwater than in the brackish water habitat. Members of the Cytophaga-Flavobacteria-Bacteroidetes group were abundant in all samples (5-35% of the total cell counts) and were especially dominant in M. spicatum samples. Alphaproteobacteria were the second major group (3-17% of the total cell counts). Betaproteobacteria, gammaproteobacteria, and actinomycetes were present in all samples (5 or 10% of the total cell counts). Planctomycetes were almost absent on M. spicatum in freshwater, but present on C. aspera in freshwater and on both plants in brackish water.
Conclusion: Bacterial biofilm communities on the surface of aquatic plants might be influenced by the host plant and environmental factors. Distinct plant species, plant part and habitat specific differences in total cell counts and two bacterial groups (CFB, planctomycetes) support the combined impact of substrate (plant) and habitat on epiphytic bacterial community composition. The presence of polyphenols might explain the distinct bacterial community on freshwater M. spicatum compared to that of M. spicatum in brackish water and of C. aspera in both habitats.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

