Morphology and function of cryopreserved whole ovine ovaries after heterotopic autotransplantation
- PMID: 18402709
- PMCID: PMC2323016
- DOI: 10.1186/1477-7827-6-16
Morphology and function of cryopreserved whole ovine ovaries after heterotopic autotransplantation
Abstract
Background: The objective of this study was to perform complex characterization of cryopreserved and then autotransplanted ovaries including determination of the ability to respond to in vivo follicle stimulating hormone (FSH)-treatment, fertilizability of retrieved oocytes, and morphology, vascularization, cellular proliferation and apoptosis in sheep.
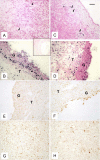
Methods: Mature crossbred ewes were divided into two groups; an intact (control) group (n = 4), and autotransplanted group (n = 4) in which oophorectomy was performed laparoscopically and ovaries with intact vascular pedicles frozen, thawed and transplanted back into the same animal at a different site. Approximately five months after autotransplantation, estrus was synchronized, ewes were treated with FSH, and ovaries were collected. For all ovaries, number of visible follicles was determined, and collected cumulus oocyte complexes (COC) were matured and fertilized in vitro. Remaining ovarian tissues were fixed for evaluation of morphology, expression of factor VIII (marker of endothelial cells), vascular endothelial growth factor (VEGF; expressed by pericytes and smooth muscle cells), and smooth muscle cell actin (SMCA; marker of pericytes and smooth muscle cells), and cellular proliferation and apoptosis. Two fully functional ovaries were collected from each control ewe (total 8 ovaries).
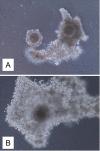
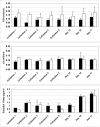
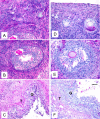
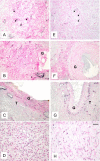
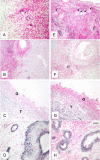
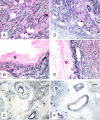
Results: Out of eight autotransplanted ovaries, a total of two ovaries with developing follicles were found. Control ewes had 10.6 +/- 2.7 follicles/ovary, oocytes were in vitro fertilized and developed to the blastocyst stage. One autotransplanted ewe had 4 visible follicles from which 3 COC were collected, but none of them was fertilized. The morphology of autotransplanted and control ovaries was similar. In control and autotransplanted ovaries, primordial, primary, secondary, antral and preovulatory follicles were found along with fully functional vascularization which was manifested by expression of factor VIII, VEGF and SMCA. Proliferating cells were detected in follicles, and the rate of apoptosis was minimal in ovaries of control and autotransplanted ovaries.
Conclusion: These data demonstrate successful autotransplantation of a portion of frozen/thawed ovaries manifested by restoration of selected ovarian function including in vitro maturation of collected oocytes, presence of follicles from several stages of folliculogenesis and blood vessels expressing specific markers of vascularization, and proliferation and apoptosis of ovarian cells. Thus, heterotopic autotransplantation of a whole frozen/thawed ovary allows for development of preovulatory follicles, oocyte growth, and for restoration of vascularization and cellular function. However, additional improvements are required to enhance the efficiency of autotransplantation of frozen/thawed ovaries to produce more oocytes.
Figures
Similar articles
-
Ovarian endocrine profile and long-term vascular patency following heterotopic autotransplantation of cryopreserved whole ovine ovaries.Hum Reprod. 2009 Nov;24(11):2845-55. doi: 10.1093/humrep/dep274. Epub 2009 Jul 29. Hum Reprod. 2009. PMID: 19640895
-
Restoration of ovarian function after autotransplantation of intact frozen-thawed sheep ovaries with microvascular anastomosis.Fertil Steril. 2003 Mar;79(3):594-602. doi: 10.1016/s0015-0282(02)04842-2. Fertil Steril. 2003. PMID: 12620446
-
Effect of resveratrol on mouse ovarian vitrification and transplantation.Reprod Biol Endocrinol. 2021 Apr 9;19(1):54. doi: 10.1186/s12958-021-00735-y. Reprod Biol Endocrinol. 2021. PMID: 33836793 Free PMC article.
-
Ovarian tissue cryopreservation and transplantation: scientific implications.J Assist Reprod Genet. 2016 Dec;33(12):1595-1603. doi: 10.1007/s10815-016-0814-1. Epub 2016 Oct 8. J Assist Reprod Genet. 2016. PMID: 27722934 Free PMC article. Review.
-
Long-term ovarian function in sheep after ovariectomy and autotransplantation of cryopreserved cortical strips.Eur J Obstet Gynecol Reprod Biol. 2004 Apr 5;113 Suppl 1:S55-9. doi: 10.1016/j.ejogrb.2003.11.023. Eur J Obstet Gynecol Reprod Biol. 2004. PMID: 15041133 Review.
Cited by
-
Blood vessel remodeling in pig ovarian follicles during the periovulatory period: an immunohistochemistry and SEM-corrosion casting study.Reprod Biol Endocrinol. 2009 Jul 16;7:72. doi: 10.1186/1477-7827-7-72. Reprod Biol Endocrinol. 2009. PMID: 19607713 Free PMC article.
-
Techniques of Cryopreservation for Ovarian Tissue and Whole Ovary.Clin Med Insights Reprod Health. 2019 Dec 3;13:1179558119884945. doi: 10.1177/1179558119884945. eCollection 2019. Clin Med Insights Reprod Health. 2019. PMID: 31839716 Free PMC article. Review.
-
Regenerative Medicine Approaches in Bioengineering Female Reproductive Tissues.Reprod Sci. 2021 Jun;28(6):1573-1595. doi: 10.1007/s43032-021-00548-9. Epub 2021 Apr 20. Reprod Sci. 2021. PMID: 33877644 Review.
-
Whole ovine ovaries as a model for human: perfusion with cryoprotectants in vivo and in vitro.Biomed Res Int. 2014;2014:409019. doi: 10.1155/2014/409019. Epub 2014 Feb 19. Biomed Res Int. 2014. PMID: 24701576 Free PMC article.
-
Whole Ovary Cryopreservation and Transplantation: A Systematic Review of Challenges and Research Developments in Animal Experiments and Humans.J Clin Med. 2020 Oct 2;9(10):3196. doi: 10.3390/jcm9103196. J Clin Med. 2020. PMID: 33023111 Free PMC article. Review.
References
-
- Mertens AC, Yasui Y, Liu Y, Stovall M, Hutchinson R, Ginsberg J, Sklar C, Robison LL. Childhood Cancer Survivor Study. Pulmonary complications in survivors of childhood and adolescent cancer. A report from the Childhood Cancer Survivor Study. Cancer. 2002;95:2431–2441. doi: 10.1002/cncr.10978. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

